Page 418 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 418
Chapter 28 Thrombocytopoiesis 339
TPO-mediated megakaryocytic progenitor proliferation and possibly
terminal megakaryocyte size determination, ploidy, and cellular
TPO maturation.
Negative Regulation of TPO Signaling
As with other receptor-mediated signaling processes, feedback mecha-
nisms exist to limit or turn off the signal once initiated to avoid
uncontrolled growth. Lnk, an adaptor protein implicated in immu-
noreceptor and cytokine receptor signaling negatively modulates
Cytoplasmic TPO signaling in megakaryocytes. Overexpression of Lnk decreases
membrane TPO-dependent megakaryocyte growth and polyploidization in
BM–derived cultures. Conversely, loss of Lnk expression by gene
targeting results in increased numbers of megakaryocytes, accentu-
P P ated megakaryocyte polyploidization, and a myeloproliferative disor-
JAK2 1 1 JAK2 der in mice. This correlates with enhanced and prolonged
15
P 591 Y Y 591 P TPO-mediated induction of STAT3, STAT5, AKT, and MAPK
signaling pathways.
2 2
Following TPO binding, the TPO receptor is internalized and
P 625 Y Y 625 P subsequently degraded. This process depends on dileucine repeats,
625
591
P 630 630 P and Tyr and Tyr within the TPO receptor cytoplasmic tail, and
Y Y
involves ubiquitinylation via the E3 ubiquitin ligase c-Cbl.
pAKT
TPO Signaling in Hematopoietic Stem Cells
pSTAT3,5 pERK1/2 PI-3K
mTOR The TPO-TPO receptor signaling system is not only important for
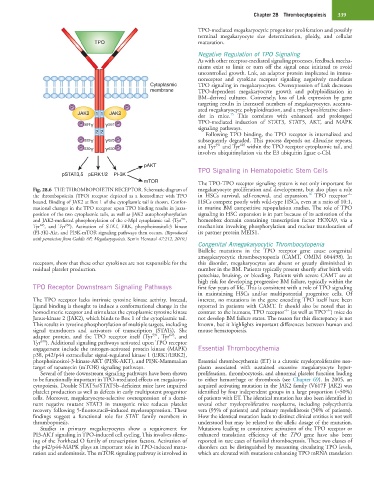
Fig. 28.6 THE THROMBOPOIETIN RECEPTOR. Schematic diagram of megakaryocyte proliferation and development, but also plays a role
−/−
16
the thrombopoietin (TPO) receptor depicted as a homodimer with TPO in HSCs survival, self-renewal, and expansion. TPO receptor
bound. Binding of JAK2 at Box 1 of the cytoplasmic tail is shown. Confor- HSCs compete poorly with wild-type HSCs, even at a ratio of 10:1,
mational changes in the TPO receptor upon TPO binding results in juxta- in murine BM competitive repopulation studies. The role of TPO
position of the two cytoplasmic tails, as well as JAK2 autophosphorylation signaling in HSC expansion is in part because of its activation of the
591
and JAK2-mediated phosphorylation of the c-Mpl cytoplasmic tail (Tyr , homeobox domain containing transcription factor HOXA9, via a
630
625
Tyr , and Tyr ). Activation of STAT, ERK, phosphoinositol-3 kinase mechanism involving phosphorylation and nuclear translocation of
(PI-3K)-Akt, and PI3K-mTOR signaling pathways then occurs. (Reproduced its partner protein MEIS1.
with permission from Geddis AE: Megakaryopoiesis. Semin Hematol 47:212, 2010.)
Congenital Amegakaryocytic Thrombocytopenia
Biallelic mutations in the TPO receptor gene cause congenital
amegakaryocytic thrombocytopenia (CAMT, OMIM 604498). In
receptors, show that these other cytokines are not responsible for the this disorder, megakaryocytes are absent or greatly diminished in
residual platelet production. number in the BM. Patients typically present shortly after birth with
petechiae, bruising, or bleeding. Patients with severe CAMT are at
high risk for developing progressive BM failure, typically within the
TPO Receptor Downstream Signaling Pathways first few years of life. This is consistent with a role of TPO signaling
in maintaining HSCs and/or multipotential progenitor cells. Of
The TPO receptor lacks intrinsic tyrosine kinase activity. Instead, interest, no mutations in the gene encoding TPO itself have been
ligand binding is thought to induce a conformational change in the reported in patients with CAMT. It should also be noted that in
−/−
−/−
homodimeric receptor and stimulates the cytoplasmic tyrosine kinase contrast to the humans, TPO receptor (as well as TPO ) mice do
Janus-kinase 2 (JAK2), which binds to Box 1 of the cytoplasmic tail. not develop BM failure states. The reason for this discrepancy is not
This results in tyrosine phosphorylation of multiple targets, including known, but it highlights important differences between human and
signal transducers and activators of transcription (STATs), Shc mouse hematopoiesis.
591
625
adaptor protein, and the TPO receptor itself (Tyr , Tyr , and
630
Tyr ). Additional signaling pathways activated upon TPO receptor
engagement include the mitogen-activated protein kinase (MAPK) Essential Thrombocythemia
p38, p42/p44 extracellular signal-regulated kinase 1 (ERK1/ERK2),
phosphoinositol-3-kinase-AKT (PI3K-AKT), and PI3K-Mammalian Essential thrombocythemia (ET) is a chronic myeloproliferative neo-
target of rapamycin (mTOR) signaling pathways. plasm associated with sustained excessive megakaryocyte hyper-
Several of these downstream signaling pathways have been shown proliferation, thrombocytosis, and abnormal platelet function leading
to be functionally important in TPO-mediated effects on megakaryo- to either hemorrhage or thrombosis (see Chapter 69). In 2005, an
cytopoiesis. Double STAT5a/STAT5b–deficient mice have impaired acquired activating mutation in the JAK2 family (V617F JAK2) was
platelet production as well as defects in early multipotent progenitor identified by four independent groups in a large proportion (≈50%)
cells. Moreover, megakaryocyte-selective overexpression of a domi- of patients with ET. The identical mutation has also been identified in
nant negative mutant STAT3 in transgenic mice reduces platelet several other myeloproliferative neoplasms, including polycythemia
recovery following 5-fluorouracil–induced myelosuppression. These vera (95% of patients) and primary myelofibrosis (50% of patients).
findings suggest a functional role for STAT family members in How the identical mutation leads to distinct clinical entities is not well
thrombopoiesis. understood but may be related to the allelic dosage of the mutation.
Studies in primary megakaryocytes show a requirement for Mutations leading to constitutive activation of the TPO receptor or
PI3-AKT signaling in TPO-induced cell cycling. This involves silenc- enhanced translation efficiency of the TPO gene have also been
ing of the Forkhead O family of transcription factors. Activation of reported in rare cases of familial thrombocytosis. These two classes of
the p42/p44-MAPK plays an important role in TPO-induced matu- disorders can be distinguished by measuring circulating TPO levels,
ration and endomitosis. The mTOR signaling pathway is involved in which are elevated with mutations enhancing TPO mRNA translation