Page 417 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 417
338 Part IV Disorders of Hematopoietic Cell Development
megakaryocytes with BM stromal cells inhibits megakaryocyte dif-
VE-cadherin ferentiation. These mechanisms are likely in place to coordinate
Vascular niche
terminal megakaryocyte maturation with vascular access, facilitating
Sinusoidal Platelets the efficient delivery of platelets into circulation.
vessel
CYTOKINE REGULATION OF THROMBOCYTOPOIESIS
Progenitors
FGF-4. SDF-1
Thrombopoietin Signaling
Thrombopoietin
TPO Physiologic stress
Stem cell (marrow suppression) It has been estimated that an adult human produces nearly 2 × 10
11
Osteoblastic niche platelets per day, and this number can increase fourfold to eightfold
11
during times of increased demand. The regulation of this process
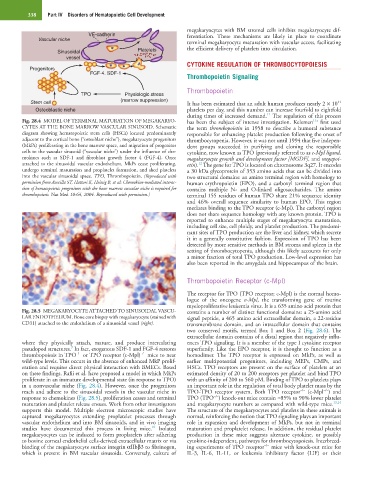
Fig. 28.4 MODEL OF TERMINAL MATURATION OF MEGAKARYO- has been the subject of intense investigation. Kelemen first used
11a
CYTES AT THE BONE MARROW VASCULAR SINUSOID. Schematic the term thrombopoietin in 1958 to describe a humoral substance
diagram showing hematopoietic stem cells (HSCs) located predominantly responsible for enhancing platelet production following the onset of
adjacent to the cortical bone (“osteoblast niche”), megakaryocyte progenitors thrombocytopenia. However, it was not until 1994 that five indepen-
(MkPs) proliferating in the bone marrow space, and migration of progenitor dent groups succeeded in purifying and cloning the responsible
cells to the vascular sinusoid (“vascular niche”) under the influence of che- cytokine, now known as TPO (previously referred to as c-Mpl ligand,
mokines such as SDF-1 and fibroblast growth factor 4 (FGF-4). Once megakaryocyte growth and development factor [MGDF], and megapoi-
attached to the sinusoidal vascular endothelium, MkPs cease proliferating, etin). The gene for TPO is located on chromosome 3q27. It encodes
12
undergo terminal maturation and proplatelet formation, and shed platelets a 30 kDa glycoprotein of 353 amino acids that can be divided into
into the vascular sinusoidal space. TPO, Thrombopoietin. (Reproduced with two structural domains: an amino terminal region with homology to
permission from Avecilla ST, Hattori K, Heissig B, et al: Chemokine-mediated interac- human erythropoietin (EPO), and a carboxyl terminal region that
tion of hematopoietic progenitors with the bone marrow vascular niche is required for contains multiple N- and O-linked oligosaccharides. The amino
thrombopoiesis. Nat Med 10:64, 2004. Reproduced with permission.) terminal 155 residues of human TPO share 21% sequence identity
and 46% overall sequence similarity to human EPO. This region
mediates binding to the TPO receptor (c-Mpl). The carboxyl region
does not share sequence homology with any known protein. TPO is
reported to enhance multiple stages of megakaryocyte maturation,
including cell size, cell ploidy, and platelet production. The predomi-
nant sites of TPO production are the liver and kidney, which secrete
it in a generally constitutive fashion. Expression of TPO has been
detected by more sensitive methods in BM stroma and spleen in the
setting of thrombocytopenia, although this likely accounts for only
a minor fraction of total TPO production. Low-level expression has
also been reported in the amygdala and hippocampus of the brain.
Thrombopoietin Receptor (c-Mpl)
The receptor for TPO (TPO receptor; c-Mpl) is the normal homo-
logue of the oncogene v-Mpl, the transforming gene of murine
myeloproliferative leukemia virus. It is a 635 amino acid protein that
Fig. 28.5 MEGAKARYOCYTE ATTACHED TO SINUSOIDAL VASCU- contains a number of distinct functional domains: a 25-amino acid
LAR ENDOTHELIUM. Bone core biopsy with megakaryocyte (stained with signal peptide, a 465 amino acid extracellular domain, a 22-residue
CD31) attached to the endothelium of a sinusoidal vessel (right). transmembrane domain, and an intracellular domain that contains
two conserved motifs, termed Box 1 and Box 2 (Fig. 28.6). The
extracellular domain contains of a distal region that negatively influ-
where they physically attach, mature, and produce intercalating ences TPO signaling. It is a member of the type I cytokine receptor
9
pseudopod structures. In fact, exogenous SDF-1 and FGF-4 restores superfamily. Like the EPO receptor, it is thought to function as a
−/−
−/−
thrombopoiesis in TPO or TPO receptor (c-Mpl) mice to near homodimer. The TPO receptor is expressed on MkPs, as well as
wild-type levels. This occurs in the absence of enhanced MkP prolif- earlier multipotential progenitors, including MEPs, CMPs, and
eration and requires direct physical interaction with BMECs. Based HSCs. TPO receptors are present on the surface of platelets at an
on these findings, Rafii et al. have proposed a model in which MkPs estimated density of 20 to 200 receptors per platelet and bind TPO
proliferate in an immature developmental state (in response to TPO) with an affinity of 200 to 560 pM. Binding of TPO to platelets plays
in a nonvascular niche (Fig. 28.4). However, once the progenitors an important role in the regulation of total body platelet mass by the
−/−
−/−
reach and adhere to the sinusoidal vessels in the vascular niche in TPO-TPO receptor system. Both TPO receptor (c-Mpl ) and
−/−
response to chemokines (Fig. 28.5), proliferation ceases and terminal TPO (TPO ) knock-out mice contain ≈85% to 90% lower platelet
maturation and platelet release ensues. Work from other investigators and megakaryocyte numbers as compared with wild-type mice. 13,14
supports this model. Multiple electron microscopic studies have The structure of the megakaryocytes and platelets in these animals is
captured megakaryocytes extending proplatelet processes through normal, reinforcing the notion that TPO signaling plays an important
vascular endothelium and into BM sinusoids, and in vivo imaging role in expansion and development of MkPs, but not in terminal
10
studies have documented this process in living mice. Isolated maturation and proplatelet release. In addition, the residual platelet
megakaryocytes can be induced to form proplatelets after adhering production in these mice suggests alternate cytokine, or possibly
to bovine corneal endothelial cells-derived extracellular matrix or via cytokine-independent, pathways for thrombocytopoiesis. Interbreed-
−/−
binding of the megakaryocyte surface integrin αIIbβ3 to fibrinogen, ing experiments of TPO receptor mice with knock-out mice for
which is present in BM vascular sinusoids. Conversely, culture of IL-3, IL-6, IL-11, or leukemia inhibitory factor (LIF) or their