Page 556 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 556
Chapter 35 Pathophysiology of Iron Homeostasis 471
Tf
Fe 2 Tf FLVCR
Mitochondria
TFR1 Fe 3+
Protoporhyrin IX Globin
STEAP3 Mitoferrin
Fe 2+ ?
Heme Hb
DMT1
Fe 2+ Krebs cycle Hemoproteins
enzymes Cytochromes
Cytoplasm
CI
[Fe-S]
CIII Respiratory chain
enzymes
ABC7
Ferroportin
[Fe-S]
IRP1
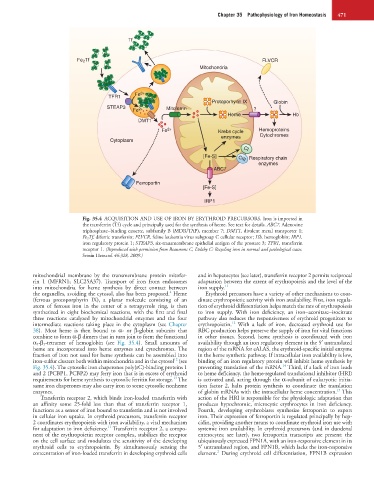
Fig. 35.4 ACQUISITION AND USE OF IRON BY ERYTHROID PRECURSORS. Iron is imported in
the transferrin (Tf) cycle and principally used for the synthesis of heme. See text for details. ABC7, Adenosine
triphosphate−binding cassette, subfamily B (MDR/TAP), member 7; DMT1, divalent metal transporter 1;
Fe 2 Tf, diferric transferrin; FLVCR, feline leukemia virus subgroup C cellular receptor; Hb, hemoglobin; IRP1,
iron regulatory protein 1; STEAP3, six-transmembrane epithelial antigen of the prostate 3; TFR1, transferrin
receptor 1. (Reproduced with permission from Beaumont C, Delaby C: Recycling iron in normal and pathological states.
Semin Hematol 46:328, 2009.)
mitochondrial membrane by the transmembrane protein mitofer- and in hepatocytes (see later), transferrin receptor 2 permits reciprocal
rin 1 (MFRN1; SLC25A37). Transport of iron from endosomes adaptation between the extent of erythropoiesis and the level of the
into mitochondria for heme synthesis by direct contact between iron supply. 11
4
the organelles, avoiding the cytosol, also has been proposed. Heme Erythroid precursors have a variety of other mechanisms to coor-
(ferrous protoporphyrin IX), a planar molecule consisting of an dinate erythropoietic activity with iron availability. First, iron regula-
atom of ferrous iron in the center of a tetrapyrrole ring, is then tion of erythroid differentiation helps match the rate of erythropoiesis
synthesized in eight biochemical reactions, with the first and final to iron supply. With iron deficiency, an iron−aconitase−isocitrate
three reactions catalyzed by mitochondrial enzymes and the four pathway also reduces the responsiveness of erythroid progenitors to
12
intermediate reactions taking place in the cytoplasm (see Chapter erythropoietin. With a lack of iron, decreased erythroid use for
38). Most heme is then bound to α- or β-globin subunits that RBC production helps preserve the supply of iron for vital functions
combine to form α-β dimers that in turn join to form the functional in other tissues. Second, heme synthesis is coordinated with iron
α 2 -β 2 -tetramer of hemoglobin (see Fig. 35.4). Small amounts of availability through an iron regulatory element in the 5′ untranslated
heme are incorporated into heme enzymes and cytochromes. The region of the mRNA for eALAS, the erythroid-specific initial enzyme
fraction of iron not used for heme synthesis can be assembled into in the heme synthetic pathway. If intracellular iron availability is low,
12
iron-sulfur clusters both within mitochondria and in the cytosol (see binding of an iron regulatory protein will inhibit heme synthesis by
14
Fig. 35.4). The cytosolic iron chaperones poly(rC)-binding proteins 1 preventing translation of the mRNA. Third, if a lack of iron leads
and 2 (PCBP1, PCBP2) may ferry iron that is in excess of erythroid to heme deficiency, the heme-regulated translational inhibitor (HRI)
13
requirements for heme synthesis to cytosolic ferritin for storage. The is activated and, acting through the α-subunit of eukaryotic initia-
same iron chaperones may also carry iron to some cytosolic nonheme tion factor 2, halts protein synthesis to coordinate the translation
15
enzymes. of globin mRNAs with the intracellular heme concentration. This
Transferrin receptor 2, which binds iron-loaded transferrin with action of the HRI is responsible for the physiologic adaptation that
an affinity some 25-fold less than that of transferrin receptor 1, produces hypochromic, microcytic erythrocytes in iron deficiency.
functions as a sensor of iron bound to transferrin and is not involved Fourth, developing erythroblasts synthesize ferroportin to export
in cellular iron uptake. In erythroid precursors, transferrin receptor iron. Their expression of ferroportin is regulated principally by hep-
2 coordinates erythropoiesis with iron availability, a vital mechanism cidin, providing another means to coordinate erythroid iron use with
11
for adaptation to iron deficiency. Transferrin receptor 2, a compo- systemic iron availability. In erythroid precursors (and in duodenal
nent of the erythropoietin receptor complex, stabilizes the receptor enterocytes; see later), two ferroportin transcripts are present: the
on the cell surface and modulates the sensitivity of the developing ubiquitously expressed FPN1A, with an iron-responsive element in its
erythroid cells to erythropoietin. By simultaneously sensing the 5′ untranslated region, and FPN1B, which lacks the iron-responsive
2
concentration of iron-loaded transferrin in developing erythroid cells element. During erythroid cell differentiation, FPN1B expression