Page 846 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 846
Chapter 52 Histiocytic Disorders 729
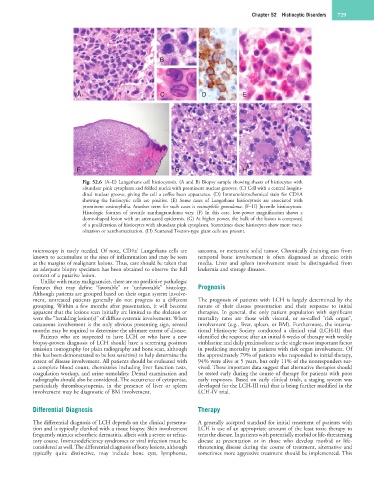
B
A C D E
F G H
Fig. 52.6 (A–E) Langerhans cell histiocytosis. (A and B) Biopsy sample showing sheets of histiocytes with
abundant pink cytoplasm and folded nuclei with prominent nuclear grooves. (C) Cell with a central longitu-
dinal nuclear groove, giving the cell a coffee bean appearance. (D) Immunohistochemical stain for CD1A
showing the histiocytic cells are positive. (E) Some cases of Langerhans histiocytosis are associated with
prominent eosinophilia. Another term for such cases is eosinophilic granuloma. (F–H) Juvenile histiocytosis.
Histologic features of juvenile xanthogranuloma vary. (F) In this case, low-power magnification shows a
dome-shaped lesion with an attenuated epidermis. (G) At higher power, the bulk of the lesion is composed
of a proliferation of histiocytes with abundant pink cytoplasm. Sometimes these histiocytes show more vacu-
olization or xanthomatization. (H) Scattered Touton-type giant cells are present.
+
microscopy is rarely needed. Of note, CD1a Langerhans cells are sarcoma, or metastatic solid tumor. Chronically draining ears from
known to accumulate at the sites of inflammation and may be seen temporal bone involvement is often diagnosed as chronic otitis
at the margins of malignant lesions. Thus, care should be taken that media. Liver and spleen involvement must be distinguished from
an adequate biopsy specimen has been obtained to observe the full leukemia and storage diseases.
context of a putative lesion.
Unlike with many malignancies, there are no predictive pathologic
features that may define “favorable” or “unfavorable” histology. Prognosis
Although patients are grouped based on their organ system involve-
ment, untreated patients generally do not progress to a different The prognosis of patients with LCH is largely determined by the
grouping. Within a few months after presentation, it will become nature of their disease presentation and their response to initial
apparent that the lesions seen initially are limited to the skeleton or therapies. In general, the only patient population with significant
were the “heralding lesion(s)” of diffuse systemic involvement. When mortality rates are those with visceral, or so-called “risk organ”,
cutaneous involvement is the only obvious presenting sign, several involvement (e.g., liver, spleen, or BM). Furthermore, the interna-
months may be required to determine the ultimate extent of disease. tional Histiocyte Society conducted a clinical trial (LCH-II) that
Patients who are suspected to have LCH or who have a new identified the response after an initial 6 weeks of therapy with weekly
biopsy-proven diagnosis of LCH should have a screening positron vinblastine and daily prednisolone as the single most important factor
emission tomography (or plain radiography and bone scan, although in predicting mortality in patients with risk organ involvement. Of
this has been demonstrated to be less sensitive) to help determine the the approximately 79% of patients who responded to initial therapy,
extent of disease involvement. All patients should be evaluated with 94% were alive at 5 years, but only 11% of the nonresponders sur-
a complete blood count, chemistries including liver function tests, vived. These important data suggest that alternative therapies should
coagulation workup, and urine osmolality. Dental examination and be tested early during the course of therapy for patients with poor
radiographs should also be considered. The occurrence of cytopenias, early responses. Based on early clinical trials, a staging system was
particularly thrombocytopenia, in the presence of liver or spleen developed for the LCH-III trial that is being further modified in the
involvement may be diagnostic of BM involvement. LCH-IV trial.
Differential Diagnosis Therapy
The differential diagnosis of LCH depends on the clinical presenta- A generally accepted standard for initial treatment of patients with
tion and is typically clarified with a tissue biopsy. Skin involvement LCH is use of an appropriate amount of the least toxic therapy to
frequently mimics seborrheic dermatitis, albeit with a severe or refrac- treat the disease. In patients with potentially morbid or life-threatening
tory course. Immunodeficiency syndromes or viral infection must be disease at presentation or in those who develop morbid or life-
considered as well. The differential diagnosis of bony lesions, although threatening disease during the course of treatment, alternative and
typically quite distinctive, may include bone cyst, lymphoma, sometimes more aggressive treatment should be implemented. This