Page 935 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 935
818 Part VII Hematologic Malignancies
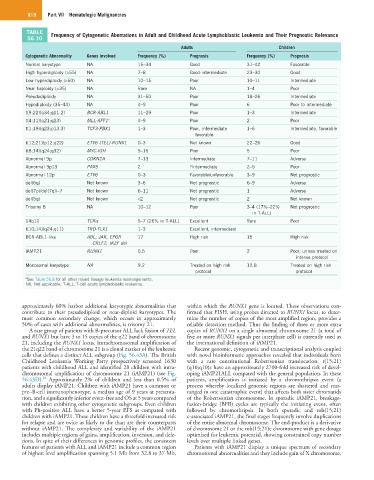
TABLE Frequency of Cytogenetic Aberrations in Adult and Childhood Acute Lymphoblastic Leukemia and Their Prognostic Relevance
56.10
Adults Children
Cytogenetic Abnormality Genes Involved Frequency (%) Prognosis Frequency (%) Prognosis
Normal karyotype NA 15–34 Good 31–42 Favorable
High hyperdiploidy (>55) NA 7–8 Good-intermediate 23–30 Good
Low hyperdiploidy (>50) NA 10–15 Poor 10–11 Intermediate
Near haploidy (<35) NA Rare NA 1–4 Poor
Pseudodiploidy NA 31–50 Poor 18–26 Intermediate
Hypodiploidy (35–44) NA 4–9 Poor 6 Poor to intermediate
t(9;22)(q34;q11.2) BCR-ABL1 11–29 Poor 1–3 Intermediate
t(4;11)(q21;q23) MLL-AFF1 a 4–9 Poor 2 Poor
t(1;19)(q23;p13.3) TCF3-PBX1 1–3 Poor, intermediate 1–6 Intermediate, favorable
favorable
t(12;21)(p12;q22) ETV6 (TEL)-RUNX1 0–3 Not known 22–26 Good
t(8;14)(q24;q32) MYC-IGH 5–15 Poor 5 Poor
Abnormal 9p CDKN2A 7–13 Intermediate 7–11 Adverse
Abnormal 9p13 PAX5 2 ?Intermediate 2–5 Poor
Abnormal 12p ETV6 0–3 Favorable/unfavorable 3–9 Not prognostic
del(6q) Not known 3–6 Not prognostic 6–9 Adverse
del(7p)/del(7q)/−7 Not known 6–11 Not prognostic 1 Adverse
del(5q) Not known <2 Not prognostic 2 Not known
Trisomy 8 NA 10–12 Poor 3–4 (17%–22% Not prognostic
in T-ALL)
14q11 TCRα 5–7 (26% in T-ALL) Excellent Rare Poor
t(10;14)(q24;q11) TRD-TLX1 1–3 Excellent, intermediate
BCR-ABL1-like ABL, JAK, EPOR 17 High risk 15 High risk
CRLF2, IKZF del
iAMP21 RUNX1 0.5 Poor 2 Poor, unless treated on
intense protocol
Monosomal karyotype NA 9.2 Treated on high risk 12.8 Treated on high risk
protocol protocol
a See Table 56.8 for all other mixed lineage leukemia rearrangements.
NA, Not applicable; T-ALL, T-cell acute lymphoblastic leukemia.
approximately 60% harbor additional karyotypic abnormalities that within which the RUNX1 gene is located. These observations con-
contribute to their pseudodiploid or near-diploid karyotypes. The firmed that FISH, using probes directed to RUNX1 locus, to deter-
most common secondary change, which occurs in approximately mine the number of copies of the most amplified region, provides a
50% of cases with additional abnormalities, is trisomy 21. reliable detection method. Thus the finding of three or more extra
A rare group of patients with B-precursor ALL lack fusion of TEL copies of RUNX1 on a single abnormal chromosome 21 (a total of
and RUNX1 but have 3 to 15 copies of the q22 band of chromosome five or more RUNX1 signals per interphase cell) is currently used as
21, including the RUNX1 locus. Intrachromosomal amplification of the international definition of iAMP21.
the 21q22 band of chromosome 21 is a clonal marker of the leukemic Recent genomic, cytogenetic and transcriptional analysis coupled
cells that defines a distinct ALL subgroup (Fig. 56.43A). The British with novel bioinformatic approaches revealed that individuals born
Childhood Leukemia Working Party prospectively screened 1630 with a rare constitutional Robertsonian translocation, t(15;21)
patients with childhood ALL and identified 28 children with intra- (q10;q10)c have an approximately 2700-fold increased risk of devel-
chromosomal amplification of chromosome 21 (iAMP21) (see Fig. oping iAMP21ALL compared with the general population. In these
24
56.43D). Approximately 2% of children and less than 0.5% of patients, amplification is initiated by a chromothripsis event (a
adults display iAMP21. Children with iAMP21 have a common or process whereby localized genomic regions are shattered and rear-
pre–B-cell immunophenotype, a median age of 9 years at presenta- ranged in one catastrophic event) that affects both sister chromatids
tion, and a significantly inferior event-free and OS at 5 years compared of the Robertsonian chromosome. In sporadic iAMP21, breakage-
with children exhibiting other cytogenetic subgroups. Even children fusion-bridge (BFB) cycles are typically the initiating event, often
with Ph-positive ALL have a better 5-year EFS as compared with followed by chromothripsis. In both sporadic and rob(15;21)
children with iAMP21. These children have a threefold increased risk c-associated iAMP21, the final stages frequently involve duplications
for relapse and are twice as likely to die than are their counterparts of the entire abnormal chromosome. The end-product is a derivative
without iAMP21. The complexity and variability of the iAMP21 of chromosome 21 or the rob(15;21)c chromosome with gene dosage
includes multiple regions of gains, amplification, inversion, and dele- optimized for leukemic potential, showing constrained copy number
tions. In spite of their differences in genomic profiles, the consistent levels over multiple linked genes.
features of patients with ALL and iAMP21 include a common region Patients with iAMP21 display a unique spectrum of secondary
of highest level amplification spanning 5.1 Mb from 32.8 to 37 Mb, chromosomal abnormalities and they include gain of X chromosome,