Page 1094 - Williams Hematology ( PDFDrive )
P. 1094
1068 Part VIII: Monocytes and Macrophages Chapter 67: Structure, Receptors, and Functions of Monocytes and Macrophages 1069
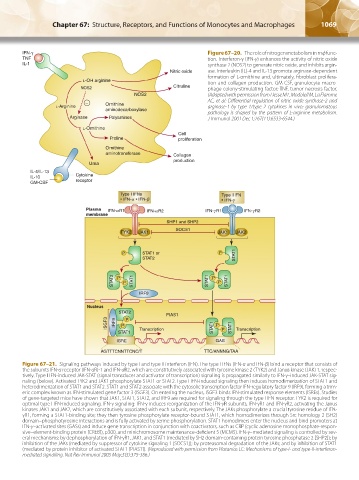
IFN-γ Figure 67–20. The role of nitrogen metabolism in mф func-
TNF tion. Interferon-γ (IFN-γ) enhances the activity of nitric oxide
IL-1 synthase 2 (NOS2) to generate nitric oxide, and inhibits argin-
Nitric oxide ase. Interleukin (IL)-4 and IL-13 promote arginase-dependent
formation of L-ornithine and, ultimately, fibroblast prolifera-
L-OH arginine tion and collagen production. GM-CSF, granulocyte-macro-
NOS2 Citruline phage colony-stimulating factor; TNF, tumor necrosis factor.
NOS2 (Adapted with permission from Hesse M1, Modolell M, La Flamme
– Ornithine AC, et al: Differential regulation of nitric oxide synthase-2 and
L-Arginine arginase-1 by type 1/type 2 cytokines in vivo: granulomatous
aminodecarboxylase
pathology is shaped by the pattern of L-arginine metabolism.
Arginase Polyamines J Immunol 2001 Dec 1;167(11):6533-6544.)
L-Ornithine
Cell
Proline proliferation
Ornithine
aminotransferase Collagen
production
Urea
IL-4/IL-13
IL-10 Cytokine
GM-CSF receptor
Figure 67–21. Signaling pathways induced by type I and type II interferon (IFN). The type I IFNs (IFN-α and IFN-β) bind a receptor that consists of
the subunits IFN-α receptor (IFN-αR)-1 and IFN-αR2, which are constitutively associated with tyrosine kinase 2 (TYK2) and Janus kinase (JAK) 1, respec-
tively. Type I IFN-induced JAK-STAT (signal transducer and activator of transcription) signaling is propagated similarly to IFN-γ–induced JAK-STAT sig-
naling (below). Activated TYK2 and JAK1 phosphorylate STAT1 or STAT2. Type I IFN-induced signaling then induces homodimerization of STAT1 and
heterodimerization of STAT1 and STAT2. STAT1 and STAT2 associate with the cytosolic transcription factor IFN-regulatory factor 9 (IRF9), forming a trim-
eric complex known as IFN-stimulated gene factor 3 (ISGF3). On entering the nucleus, ISGF3 binds IFN-stimulated response elements (ISREs). Studies
of gene-targeted mice have shown that JAK1, STAT1, STAT2, and IRF9 are required for signaling through the type I IFN receptor. TYK2 is required for
optimal type I IFN-induced signaling. IFN-γ signaling: IFN-γ induces reorganization of the IFN-γR subunits, IFN-γR1 and IFN-γR2, activating the Janus
kinases JAK1 and JAK2, which are constitutively associated with each subunit, respectively. The JAKs phosphorylate a crucial tyrosine residue of IFN-
γR1, forming a STAT1-binding site; they then tyrosine phosphorylate receptor-bound STAT1, which homodimerizes through Src homology 2 (SH2)
domain–phosphotyrosine interactions and is fully activated by serine phosphorylation. STAT1 homodimers enter the nucleus and bind promoters at
IFN-γ–activated sites (GASs) and induce gene transcription in conjunction with coactivators, such as CBP (cyclic adenosine monophosphate-respon-
sive–element-binding protein [CREB]), p300, and minichromosome maintenance-deficient 5 (MCM5). IFN-γ–mediated signaling is controlled by sev-
eral mechanisms: by dephosphorylation of IFN-γR1, JAK1, and STAT1 (mediated by SH2 domain-containing protein tyrosine phosphatase 2 [SHP2]); by
inhibition of the JAKs (mediated by suppressor of cytokine signaling 1 [SOCS1]); by proteasomal degradation of the JAKs; and by inhibition of STAT1
(mediated by protein inhibitor of activated STAT1 [PIAS1]). (Reproduced with permission from Platanias LC: Mechanisms of type-I- and type-II-interferon-
mediated signalling. Nat Rev Immunol 2005 May;5(5):375-386.)
Kaushansky_chapter 67_p1043-1074.indd 1069 9/21/15 10:44 AM