Page 1093 - Williams Hematology ( PDFDrive )
P. 1093
1068 Part VIII: Monocytes and Macrophages Chapter 67: Structure, Receptors, and Functions of Monocytes and Macrophages 1069
NADPH Bacterium
oxidase
complex 2O 2
p22 – . Cl – HOCl Glucose + NADP + Pentose-P
p47 2O 2 O 2
p40 gp91 H O H + G6PD + NADPH
2 2
+ MPO
NADPH 2 p67
2e – Rac NADPH + O 2 NADP +
2H + + O
Cytochrome b 558 2
– +
2O 2 + 2H H O + O –
1
Proton Superoxide dismutase 2 2 2
+ +
NADPH + 2H channel – – 1
2O 2 + H O 2 .OH + OH + O 2
2
A
H O + Cl – –
2
2
2
Myeloperoxidase OCl + H O
–
–
OCL + H O 1 O + Cl + H O
2
2
2
NADPH Bacterium
–
oxidase 2O + 2H +
2
1
complex 2O 2 Superoxide dismutase H 2 O + O 2
2
p22 – .
p47 2O 2 2OH – pH
2
p40 gp91 2H O 2
+ H O + O 2
2
NADPH 2 p67 Catalase
2e – Rac C
Hypertonic
Potassium
+ + + channel
NADP + 2H pH K
B
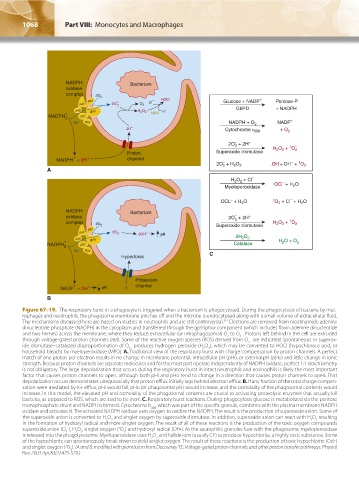
Figure 67–19. The respiratory burst in a phagocyte is triggered when a bacterium is phagocytosed. During the phagocytosis of bacteria by mac-
rophages and neutrophils, the phagosome membrane pinches off and the microbe is endocytosed along with a small volume of extracellular fluid.
112
The mechanisms discussed here are based on studies in neutrophils and are still controversial. Electrons are removed from nicotinamide adenine
dinucleotide phosphate (NADPH) in the cytoplasm and transferred through the gp91phox component (which includes flavin adenine dinucleotide
–
and two hemes) across the membrane, where they reduce extracellular (or intraphagosomal) O to O . Protons left behind in the cell are extruded
2
2
–
through voltage-gated proton channels (red). Some of the reactive oxygen species (ROS) derived from O are indicated. Spontaneous or superox-
2
–
ide dismutase–catalyzed disproportionation of O produces hydrogen peroxide (H O ), which may be converted to HOCl (hypochlorous acid, or
2
2
2
household bleach) by myeloperoxidase (MPO). A. Traditional view of the respiratory burst with charge compensation by proton channels. A perfect
match of one proton per electron results in no change in membrane potential, intracellular pH (pHi), or external pH (pHo) and little change in ionic
strength. Because proton channels are separate molecules and for the most part operate independently of NADPH oxidase, perfect 1:1 stoichiometry
is not obligatory. The large depolarization that occurs during the respiratory burst in intact neutrophils and eosinophils is likely the most important
factor that causes proton channels to open, although both pHi and pHo tend to change in a direction that causes proton channels to open. That
depolarization occurs demonstrates unequivocally that proton efflux initially lags behind electron efflux. B. If any fraction of the total charge compen-
sation were mediated by K+ efflux, pHi would fall, pHo (or phagosomal pH) would increase, and the osmolality of the phagosomal contents would
increase. In this model, the elevated pH and osmolality of the phagosomal contents are crucial to activating proteolytic enzymes that actually kill
bacteria, as opposed to ROS, which are said to be inert. C. Respiratory burst reactions. During phagocytosis glucose is metabolized via the pentose
monophosphate shunt and NADPH is formed. Cytochrome b , which was part of the specific granule, combines with the plasma membrane NADPH
588
oxidase and activates it. The activated NADPH oxidase uses oxygen to oxidize the NADPH. The result is the production of superoxide anion. Some of
the superoxide anion is converted to H O and singlet oxygen by superoxide dismutase. In addition, superoxide anion can react with H O resulting
2
2
2
2
in the formation of hydroxyl radical and more singlet oxygen. The result of all of these reactions is the production of the toxic oxygen compounds
1
–
superoxide anion (O ), H O , singlet oxygen ( O ) and hydroxyl radical (OH•). As the azurophilic granules fuse with the phagosome, myeloperoxidase
2
2
2
2
–
is released into the phagolysosome. Myeloperoxidase uses H O and halide ions (usually Cl ) to produce hypochlorite, a highly toxic substance. Some
2
2
–
of the hypochlorite can spontaneously break down to yield singlet oxygen. The result of these reactions is the production of toxic hypochlorite (Ocl )
1
and singlet oxygen ( O ). (A and B, modified with permission from Decoursey TE: Voltage-gated proton channels and other proton transfer pathways, Physiol
2
Rev 2003 Apr;83(2):475-579.)
Kaushansky_chapter 67_p1043-1074.indd 1068 9/21/15 10:44 AM