Page 1090 - Williams Hematology ( PDFDrive )
P. 1090
1064 Part VIII: Monocytes and Macrophages Chapter 67: Structure, Receptors, and Functions of Monocytes and Macrophages 1065
Nutrient depletion
Erythrocyte
Induction of
autophagy
Proteasome SEC61 H H RAB11 Autophagosome
RAB4/
Heme Recycle
Fe 2+
HO
TAP Fusion with
HO lysosome
Fe 2+ MHC Degradation
Ferritin class I MHC
class II
A B C
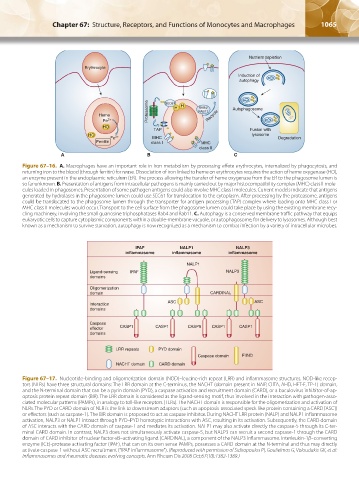
Figure 67–16. A. Macrophages have an important role in iron metabolism by processing effete erythrocytes, internalized by phagocytosis, and
returning iron to the blood (through ferritin) for reuse. Dissociation of iron linked to heme on erythrocytes requires the action of heme oxygenase (HO),
an enzyme present in the endoplasmic reticulum (ER). The process allowing the transfer of heme oxygenase from the ER to the phagosome lumen is
so far unknown. B. Presentation of antigens from intracellular pathogens is mainly carried out by major histocompatibility complex (MHC) class II mole-
cules loaded in phagosomes. Presentation of some pathogen antigens could also involve MHC class I molecules. Current models indicate that antigens
generated by hydrolases in the phagosome lumen could use SEC61 for translocation to the cytoplasm. After processing by the proteasome, antigens
could be translocated to the phagosome lumen through the transporter for antigen processing (TAP) complex where loading onto MHC class I or
MHC class II molecules would occur. Transport to the cell surface from the phagosome lumen could take place by using the existing membrane recy-
cling machinery, involving the small guanosine triphosphatases Rab4 and Rab11. C. Autophagy is a conserved membrane traffic pathway that equips
eukaryotic cells to capture cytoplasmic components within a double-membrane vacuole, or autophagosome, for delivery to lysosomes. Although best
known as a mechanism to survive starvation, autophagy is now recognized as a mechanism to combat infection by a variety of intracellular microbes.
IPAF NALP1 NALP3
inflammasome inflammasome inflammasome
NALP1
Ligand-sensing IPAF NALP3
domains
Oligomerization
domain CARDINAL
Interaction ASC ASC
domains
Caspase
effector CASP1 CASP1 CASP5 CASP1 CASP1
domains
LRR repeats PYD domain
Caspase domain FIIND
NACHT domain CARD domain
Figure 67–17. Nucleotide-binding and oligomerization domain (NOD)–leucine-rich repeat (LRR) and inflammasome structures. NOD-like recep-
tors (NLRs) have three structural domains: The LRR domain at the C-terminus, the NACHT (domain present in NAIP, CIITA, AHD, HET-E, TP-1) domain,
and the N-terminal domain that can be a pyrin domain (PYD), a caspase activation and recruitment domain (CARD), or a baculovirus inhibitor-of-ap-
optosis protein repeat domain (BIR). The LRR domain is considered as the ligand-sensing motif, thus involved in the interaction with pathogen-asso-
ciated molecular patterns (PAMPs), in analogy to toll-like receptors (TLRs). The NACHT domain is responsible for the oligomerization and activation of
NLRs. The PYD or CARD domain of NLR is the link to downstream adaptors (such as apoptosis-associated speck-like protein containing a CARD [ASC])
or effectors (such as caspase-1). The BIR domain is proposed to act as caspase inhibitor. During NACHT LRR protein (NALP) and NALP1 inflammasome
activation, NALP3 or NALP1 interact through PYD–PYD homotypic interactions with ASC, resulting in its activation. Subsequently, the CARD domain
of ASC interacts with the CARD domain of caspase-1 and mediates its activation. NALP1 may also activate directly the caspase-5 through its C-ter-
minal CARD domain. In contrast, NALP3 does not simultaneously activate caspase-5, but NALP3 can recruit a second capsase-1 through the CARD
domain of CARD inhibitor of nuclear factor-κB–activating ligand (CARDINAL), a component of the NALP3 inflammasome. Interleukin-1β–converting
enzyme (ICE)-protease activating factor (IPAF), that can on its own sense PAMPs, possesses a CARD domain at the N-terminal and thus may directly
activate caspase-1 without ASC recruitment (“IPAF inflammasome”). (Reproduced with permission of Sidiropoulos PI, Goulielmos G, Voloudakis GK, et al:
Inflammasomes and rheumatic diseases: evolving concepts. Ann Rheum Dis 2008 Oct;67(10):1382-1389.)
Kaushansky_chapter 67_p1043-1074.indd 1065 9/21/15 10:43 AM