Page 1605 - Williams Hematology ( PDFDrive )
P. 1605
1580 Part XI: Malignant Lymphoid Diseases Chapter 95: General Considerations for Lymphomas 1581
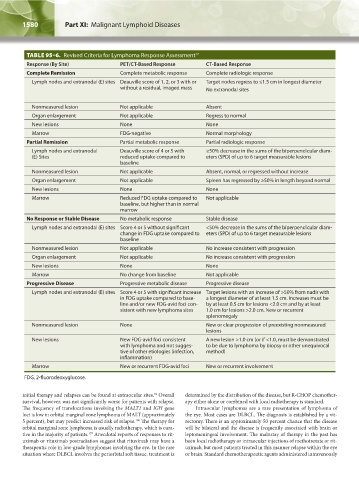
TABLE 95–6. Revised Criteria for Lymphoma Response Assessment 17
Response (By Site) PET/CT-Based Response CT-Based Response
Complete Remission Complete metabolic response Complete radiologic response
Lymph nodes and extranodal (E) sites Deauville score of 1, 2, or 3 with or Target nodes regress to ≤1.5 cm in longest diameter
without a residual, imaged mass No extranodal sites
Nonmeasured lesion Not applicable Absent
Organ enlargement Not applicable Regress to normal
New lesions None None
Marrow FDG-negative Normal morphology
Partial Remission Partial metabolic response Partial radiologic response
Lymph nodes and extranodal Deauville score of 4 or 5 with ≥50% decrease in the sums of the biperpendicular diam-
(E) Sites reduced uptake compared to eters (SPD) of up to 6 target measurable lesions
baseline
Nonmeasured lesion Not applicable Absent, normal, or regressed without increase
Organ enlargement Not applicable Spleen has regressed by ≥50% in length beyond normal
New lesions None None
Marrow Reduced FDG uptake compared to Not applicable
baseline, but higher than in normal
marrow
No Response or Stable Disease No metabolic response Stable disease
Lymph nodes and extranodal (E) sites Score 4 or 5 without significant <50% decrease in the sums of the biperpendicular diam-
change in FDG uptake compared to eters (SPD) of up to 6 target measurable lesions
baseline
Nonmeasured lesion Not applicable No increase consistent with progression
Organ enlargement Not applicable No increase consistent with progression
New lesions None None
Marrow No change from baseline Not applicable
Progressive Disease Progressive metabolic disease Progressive disease
Lymph nodes and extranodal (E) sites Score 4 or 5 with significant increase Target lesions with an increase of >50% from nadir with
in FDG uptake compared to base- a longest diameter of at least 1.5 cm. Increases must be
line and/or new FDG-avid foci con- by at least 0.5 cm for lesions <2.0 cm and by at least
sistent with new lymphoma sites 1.0 cm for lesions >2.0 cm. New or recurrent
splenomegaly
Nonmeasured lesion None New or clear progression of preexisting nonmeasured
lesions
New lesions New FDG-avid foci consistent A new lesion >1.0 cm (or if <1.0, must be demonstrated
with lymphoma and not sugges- to be due to lymphoma by biopsy or other unequivocal
tive of other etiologies (infection, method)
inflammation)
Marrow New or recurrent FDG-avid foci New or recurrent involvement
FDG, 2-fluorodeoxyglucose.
initial therapy and relapses can be found at extraocular sites. Overall determined by the distribution of the disease, but R-CHOP chemother-
91
survival, however, was not significantly worse for patients with relapse. apy either alone or combined with local radiotherapy is standard.
The frequency of translocations involving the MALT1 and IGH gene Intraocular lymphomas are a rare presentation of lymphoma of
loci is low in orbital marginal zone lymphoma of MALT (approximately the eye. Most cases are DLBCL. The diagnosis is established by a vit-
5 percent), but may predict increased risk of relapse. The therapy for rectomy. There is an approximately 50 percent chance that the disease
160
orbital marginal zone lymphoma is usually radiotherapy, which is cura- will be bilateral and the disease is frequently associated with brain or
tive in the majority of patients. Anecdotal reports of responses to rit- leptomeningeal involvement. The mainstay of therapy in the past has
157
uximab or rituximab postradiation suggest that rituximab may have a been local radiotherapy or intraocular injections of methotrexate or rit-
therapeutic role in low-grade lymphomas involving the eye. In the rare uximab, but most patients treated in this manner relapse within the eye
situation where DLBCL involves the periorbital soft tissue, treatment is or brain. Standard chemotherapeutic agents administered intravenously
Kaushansky_chapter 95_p1569-1586.indd 1580 9/21/15 12:17 PM