Page 1997 - Williams Hematology ( PDFDrive )
P. 1997
1972 Part XII: Hemostasis and Thrombosis Chapter 115: Vascular Function In Hemostasis 1973
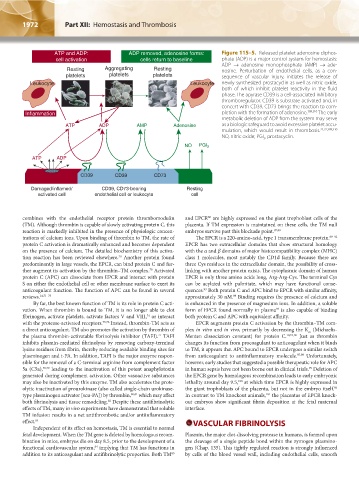
ATP and ADP: ADP removed, adenosine forms: Figure 115–5. Released platelet adenosine diphos-
cell activation cells return to baseline phate (ADP) is a major control system for hemostasis:
ADP → adenosine monophosphate (AMP) → ade-
Resting Aggregating Resting nosine. Perturbation of endothelial cells, as a con-
platelets platelets platelets sequence of vascular injury, initiates the release of
Leukocyte Leukocyte newly synthesized prostacyclin as well as nitric oxide,
both of which inhibit platelet reactivity in the fluid
phase. The apyrase CD39 is a cell-associated inhibitory
thromboregulator. CD39 is substrate-activated and, in
concert with CD39, CD73 brings the reaction to com-
Inflammation pletion with the formation of adenosine. 309,310 The early
metabolic deletion of ADP from the system may serve
ATP ADP AMP Adenosine as a biologic safeguard to avoid excessive platelet accu-
mulation, which would result in thrombosis. 21,22,309,310
NO, nitric oxide; PGI , prostacyclin.
2
NO PGI 2
ATP ADP
CD39 CD39 CD73
Damaged/inflamed/ CD39, CD73-bearing Resting
activated cell endothelial cell or leukocyte cell
combines with the endothelial receptor protein thrombomodulin and EPCR are highly expressed on the giant trophoblast cells of the
86
(TM). Although thrombin is capable of slowly activating protein C, this placenta. If TM expression is maintained on these cells, the TM null
reaction is markedly inhibited in the presence of physiologic concen- embryos survive past this blockade point. 87,88
trations of calcium ions. Upon binding of thrombin to TM, the rate of The EPCR is a 220-amino-acid, type 1 transmembrane protein. 89–92
protein C activation is dramatically enhanced and becomes dependent EPCR has two extracellular domains that show structural homology
on the presence of calcium. The detailed biochemistry of this activa- with the α and β domains of major histocompatibility complex (MHC)
tion reaction has been reviewed elsewhere. Another protein found class 1 molecules, most notably the CD1d family. Because there are
70
predominantly in large vessels, the EPCR, can bind protein C and fur- three Cys residues in the extracellular domain, the possibility of cross-
ther augment its activation by the thrombin–TM complex. Activated linking with another protein exists. The cytoplasmic domain of human
70
protein C (APC) can dissociate from EPCR and interact with protein EPCR is only three amino acids long, Arg-Arg-Cys. The terminal Cys
S on either the endothelial cell or other membrane surface to exert its can be acylated with palmitate, which may have functional conse-
anticoagulant function. The function of APC can be found in several quences. Both protein C and APC bind to EPCR with similar affinity,
93
reviews. 14,71–73 approximately 30 nM. Binding requires the presence of calcium and
89
By far, the best known function of TM is its role in protein C acti- is enhanced in the presence of magnesium ions. In addition, a soluble
vation. When thrombin is bound to TM, it is no longer able to clot form of EPCR found normally in plasma is also capable of binding
94
fibrinogen, activate platelets, activate factors V and VIII, or interact both protein C and APC with equivalent affinity.
74
with the protease-activated receptors. 75,76 Instead, thrombin-TM acts as EPCR augments protein C activation by the thrombin–TM com-
a direct anticoagulant. TM also promotes the activation by thrombin of plex in vitro and in vivo, primarily by decreasing the K (Michaelis-
m
the plasma thrombin-activatable fibrinolysis inhibitor (TAFI). TAFI Menten dissociation constant) for protein C. 70,95,96 Just as thrombin
77
inhibits plasmin-mediated fibrinolysis by removing carboxy-terminal changes its function from procoagulant to anticoagulant when it binds
lysine residues from fibrin, thereby reducing available binding sites for to TM, it appears that APC bound to EPCR undergoes a similar switch
plasminogen and t-PA. In addition, TAFI is the major enzyme respon- from anticoagulant to antiinflammatory molecule. 97,98 Unfortunately,
sible for the removal of a C-terminal arginine from complement factor however, early studies that suggested a possible therapeutic role for APC
5a (C5a), 78,79 leading to the inactivation of this potent anaphylotoxin in human sepsis have not been borne out in clinical trials. Deletion of
99
generated during complement activation. Other vasoactive substances the EPCR gene by homologous recombination leads to early embryonic
may also be inactivated by this enzyme. TM also accelerates the prote- lethality around day 9.5, at which time EPCR is highly expressed in
100
olytic inactivation of prourokinase (also called single-chain urokinase- the giant trophoblasts of the placenta, but not in the embryo itself.
86
type plasminogen activator [scu-PA]) by thrombin, 80,81 which may affect In contrast to TM knockout animals, the placentas of EPCR knock-
101
both fibrinolysis and tissue remodeling. Despite these antifibrinolytic out embryos show significant fibrin deposition at the fetal maternal
82
effects of TM, many in vivo experiments have demonstrated that soluble interface.
TM infusion results in a net antithrombotic and/or antiinflammatory
effect. 83 VASCULAR FIBRINOLYSIS
Independent of its effect on hemostasis, TM is essential to normal
fetal development. When the TM gene is deleted by homologous recom- Plasmin, the major clot-dissolving protease in humans, is formed upon
bination in mice, embryos die on day 8.5, prior to the development of a the cleavage of a single peptide bond within the zymogen plasmino-
functional cardiovascular system, implying that TM has functions in gen (Chap. 135). This tightly regulated reaction is strongly influenced
84
addition to its anticoagulant and antifibrinolytic properties. Both TM by cells of the blood vessel wall, including endothelial cells, smooth
85
Kaushansky_chapter 115_p1967-1984.indd 1972 9/18/15 10:08 AM