Page 545 - Williams Hematology ( PDFDrive )
P. 545
520 Part VI: The Erythrocyte Chapter 35: Aplastic Anemia: Acquired and Inherited 521
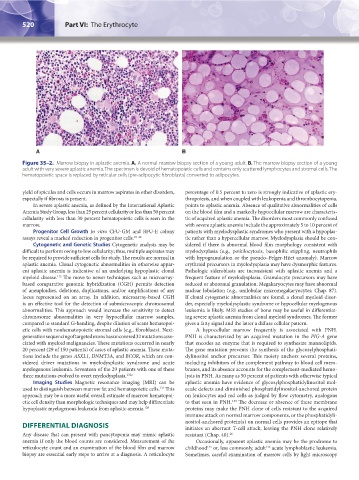
A B
Figure 35–2. Marrow biopsy in aplastic anemia. A. A normal marrow biopsy section of a young adult. B. The marrow biopsy section of a young
adult with very severe aplastic anemia. The specimen is devoid of hematopoietic cells and contains only scattered lymphocytes and stromal cells. The
hematopoietic space is replaced by reticular cells (pre-adipocytic fibroblasts) converted to adipocytes.
yield of spicules and cells occurs in marrow aspirates in other disorders, percentage of 0.5 percent to zero is strongly indicative of aplastic ery-
especially if fibrosis is present. thropoiesis, and when coupled with leukopenia and thrombocytopenia,
In severe aplastic anemia, as defined by the International Aplastic points to aplastic anemia. Absence of qualitative abnormalities of cells
Anemia Study Group, less than 25 percent cellularity or less than 50 percent on the blood film and a markedly hypocellular marrow are characteris-
cellularity with less than 30 percent hematopoietic cells is seen in the tic of acquired aplastic anemia. The disorders most commonly confused
marrow. with severe aplastic anemia include the approximately 5 to 10 percent of
Progenitor Cell Growth In vitro CFU-GM and BFU-E colony patients with myelodysplastic syndromes who present with a hypoplas-
assays reveal a marked reduction in progenitor cells. 19–22 tic rather than a hypercellular marrow. Myelodysplasia should be con-
Cytogenetic and Genetic Studies Cytogenetic analysis may be sidered if there is abnormal blood film morphology consistent with
difficult to perform owing to low cellularity; thus, multiple aspirates may myelodysplasia (e.g., poikilocytosis, basophilic stippling, neutrophils
be required to provide sufficient cells for study. The results are normal in with hypogranulation or the pseudo–Pelger-Hüet anomaly). Marrow
aplastic anemia. Clonal cytogenetic abnormalities in otherwise appar- erythroid precursors in myelodysplasia may have dysmorphic features.
ent aplastic anemia is indicative of an underlying hypoplastic clonal Pathologic sideroblasts are inconsistent with aplastic anemia and a
myeloid disease. The move to newer techniques such as microarray- frequent feature of myelodysplasia. Granulocyte precursors may have
132
based comparative genomic hybridization (CGH) permits detection reduced or abnormal granulation. Megakaryocytes may have abnormal
of aneuploidies, deletions, duplications, and/or amplifications of any nuclear lobulation (e.g., unilobular micromegakaryocytes; Chap. 87).
locus represented on an array. In addition, microarray-based CGH If clonal cytogenetic abnormalities are found, a clonal myeloid disor-
is an effective tool for the detection of submicroscopic chromosomal der, especially myelodysplastic syndrome or hypocellular myelogenous
abnormalities. This approach would increase the sensitivity to detect leukemia is likely. MRI studies of bone may be useful in differentiat-
chromosome abnormalities in very hypocellular marrow samples, ing severe aplastic anemia from clonal myeloid syndromes. The former
compared to standard G-banding, despite dilution of scant hematopoi- gives a fatty signal and the latter a diffuse cellular pattern.
etic cells with nonhematopoietic stromal cells (e.g., fibroblasts). Next- A hypocellular marrow frequently is associated with PNH.
generation sequencing of targeted exons has uncovered 32 mutations asso- PNH is characterized by an acquired mutation in the PIG-A gene
ciated with myeloid malignancies. These mutations occurred in nearly that encodes an enzyme that is required to synthesize mannolipids.
20 percent (29 of 150 patients) of cases of aplastic anemia. These muta- The gene mutation prevents the synthesis of the glycosylphosphati-
tions include the genes ASXL1, DNMT3A, and BCOR, which are con- dylinositol anchor precursor. This moiety anchors several proteins,
sidered driver mutations in myelodysplastic syndrome and acute including inhibitors of the complement pathway to blood cell mem-
myelogenous leukemia. Seventeen of the 29 patients with one of these branes, and its absence accounts for the complement-mediated hemo-
three mutations evolved to overt myelodysplasia. 132a lysis in PNH. As many as 50 percent of patients with otherwise typical
Imaging Studies Magnetic resonance imaging (MRI) can be aplastic anemia have evidence of glycosylphosphatidylinositol mol-
133
used to distinguish between marrow fat and hematopoietic cells. This ecule defects and diminished phosphatidylinositol-anchored protein
approach may be a more useful overall estimate of marrow hematopoi- on leukocytes and red cells as judged by flow cytometry, analogous
etic cell density than morphologic techniques and may help differentiate to that seen in PNH. The decrease or absence of these membrane
134
hypoplastic myelogenous leukemia from aplastic anemia. 128 proteins may make the PNH clone of cells resistant to the acquired
immune attack on normal marrow components, or the phosphatidyli-
DIFFERENTIAL DIAGNOSIS nositol-anchored protein(s) on normal cells provides an epitope that
initiates an aberrant T-cell attack, leaving the PNH clone relatively
Any disease that can present with pancytopenia may mimic aplastic resistant (Chap. 40). 26
anemia if only the blood counts are considered. Measurement of the Occasionally, apparent aplastic anemia may be the prodrome to
reticulocyte count and an examination of the blood film and marrow childhood or, less commonly, adult acute lymphoblastic leukemia.
136
135
biopsy are essential early steps to arrive at a diagnosis. A reticulocyte Sometimes, careful examination of marrow cells by light microscopy
Kaushansky_chapter 35_p0513-0538.indd 520 9/19/15 12:24 AM