Page 550 - Williams Hematology ( PDFDrive )
P. 550
524 Part VI: The Erythrocyte Chapter 35: Aplastic Anemia: Acquired and Inherited 525
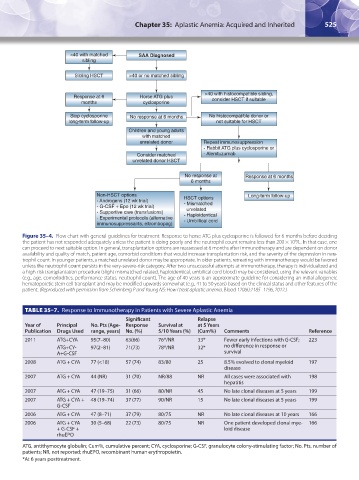
<40 with matched SAA Diagnosed
sibling
Sibling HSCT >40 or no matched sibling
>40 with histocompatible sibling,
Response at 6 Horse ATG plus consider HSCT if suitable
months cyclosporine
Stop cyclosporine No response at 6 months No histocompatible donor or
long-term follow-up not suitable for HSCT
Children and young adults
with matched
unrelated donor Repeat immunosuppression
- Rabbit ATG plus cyclosporine or
Consider matched - Alemtuzumab
unrelated donor HSCT
No response at Response at 6 months
6 months
Non-HSCT options Long-term follow-up
- Androgens (12 wk trial) HSCT options
- Mismatched
- G-CSF + Epo (12 wk trial) unrelated
- Supportive care (transfusions) - Haploidentical
- Experimental protocols (alternative - Umbillical cord
immunosuppressants, eltrombopag)
Figure 35–4. Flow chart with general guidelines for treatment. Response to horse ATG plus cyclosporine is followed for 6 months before deciding
the patient has not responded adequately unless the patient is doing poorly and the neutrophil count remains less than 200 × 10 /L. In that case, one
9
can proceed to next suitable option. In general, transplantation options are reassessed at 6 months after immunotherapy and are dependent on donor
availability and quality of match, patient age, comorbid conditions that would increase transplantation risk, and the severity of the depression in neu-
trophil count. In younger patients, a matched unrelated donor may be appropriate. In older patients, retreating with immunotherapy would be favored
unless the neutrophil count persists in the very-severe-risk category. After two unsuccessful attempts at immunotherapy, therapy is individualized and
a high-risk transplantation procedure (slight mismatched-related, haploidentical, umbilical cord blood) may be considered, using the relevant variables
(e.g., age, comorbidities, performance status, neutrophil count). The age of 40 years is an approximate guideline for considering an initial allogeneic
hematopoietic stem cell transplant and may be modified upwards somewhat (e.g., 41 to 50 years) based on the clinical status and other features of the
patient. (Reproduced with permission from Scheinberg P and Young NS: How I treat aplastic anemia. Blood 120(6):1185–1196, 2012.)
TABLE 35–7. Response to Immunotherapy in Patients with Severe Aplastic Anemia
Significant Relapse
Year of Principal No. Pts (Age- Response Survival at at 5 Years
Publication Drugs Used range, years) No. (%) 5/10 Years (%) (Cum%) Comments Reference
2011 ATG+CYA 95(7–80) 63(66) 76*/NR 33* Fewer early infections with G-CSF; 223
ATG+CY- 97(2–81) 71(73) 78*/NR 32* no difference in response or
A+G-CSF survival
2008 ATG + CYA 77 (<18) 57 (74) 83/80 25 8.5% evolved to clonal myeloid 197
disease
2007 ATG + CYA 44 (NR) 31 (70) NR/88 NR All cases were associated with 198
hepatitis
2007 ATG + CYA 47 (19–75) 31 (66) 80/NR 45 No late clonal diseases at 5 years 199
2007 ATG + CYA + 48 (19–74) 37 (77) 90/NR 15 No late clonal diseases at 5 years 199
G-CSF
2006 ATG + CYA 47 (8–71) 37 (79) 80/75 NR No late clonal diseases at 10 years 166
2006 ATG + CYA 30 (5–68) 22 (73) 80/75 NR One patient developed clonal mye- 166
+ G-CSF + loid disease
rhuEPO
ATG, antithymocyte globulin; Cum%, cumulative percent; CYA, cyclosporine; G-CSF, granulocyte colony-stimulating factor; No. Pts, number of
patients; NR, not reported; rhuEPO, recombinant human erythropoietin.
*At 6 years posttreatment.
Kaushansky_chapter 35_p0513-0538.indd 525 9/19/15 12:24 AM