Page 695 - Williams Hematology ( PDFDrive )
P. 695
670 Part VI: The Erythrocyte Chapter 46: Erythrocyte Membrane Disorders 671
TABLE 46–2. Erythrocyte Membrane Protein Defects in experienced by HS patients. The HS red cell membrane is destabilized
by a deficiency of critical membrane proteins, including spectrin,
Inherited Disorders of Red Cell Shape
ankyrin, band 3 and protein 4.2, which decreases the vertical interac-
Protein Disorder Comment tions between the skeleton and the bilayer, resulting in the release of
Ankyrin HS Most common cause of typical microvesicles and loss of surface area (Fig. 46–12). It is hypothesized
dominant HS that two mechanisms underlie the membrane loss: (1) in cells with spec-
trin/ankyrin deficiency, sections of the lipid bilayer and band 3 are not
Band 3 HS, SAO, NIHF, “Pincered” HS spherocytes seen
HAc on blood film presplenectomy; in contact with the skeleton, which will increase the lateral and rota-
SAO results from 9-amino-acid tional mobility of band 3, allowing lipid microvesicles containing band
deletion 3 to be generated, and (2) in cells with decreased amounts of band 3/
protein 4.2, the stabilizing effect of the transmembrane section of band
β-Spectrin HS, HE, HPP, “Acanthocytic” spherocytes seen 3 on the lipid bilayer is lost, facilitating the formation of band 3-free
NIHF on blood film presplenectomy; 13
location of mutation in β-spectrin microvesicles.
determines clinical phenotype
Red Cell Membrane Protein Defects
α-Spectrin HS, HE, HPP, Location of mutation in α-spectrin Analysis of HS red cell membrane proteins by several research groups
NIHF determines clinical phenotype; has revealed quantitative abnormalities of spectrin, ankyrin, band 3,
α-spectrin mutations most com- 13,63,64
mon cause of typical HE and protein 4.2 in 70 to 90 percent of the cases. This spectrum of
defects is found worldwide in all the HS cohorts that have been stud-
Protein 4.2 HS Primarily found in Japanese ied; however, the relative frequency of each defect varies with the geo-
patients
graphical area and ethnic group. In the United States, parts of Europe,
Protein 4.1 HE Found in certain European and and in Korea, the most common defect is ankyrin deficiency (30 to 60
Arab populations percent), 63,65,66 whereas it is relatively uncommon elsewhere in the world
GPC HE Concomitant protein 4.1 (<15 percent). In other parts of Europe 64,67 and in South Africa (unpub-
deficiency is basis of HE in lished), band 3 deficiency is the main defect. In Japan, almost half of the
GPC defects HS cases are caused by a decreased amount of protein 4.2, and in Korea
and South Africa, this defect is the second most common, but in other
GPC, glycophorin C; HAc, hereditary acanthocytosis; HE, hereditary populations it is rare (<6 percent). 63–65 The underlying gene mutations
elliptocytosis; HPP, hereditary pyropoikilocytosis; HS, hereditary have not been investigated in all HS subjects, but the limited research
spherocytosis; NIHF, nonimmune hydrops fetalis; SAO, southeast that has been conducted on the defective genes has identified more than
Asian ovalocytosis.
140 different mutations, which are often unique to a family.
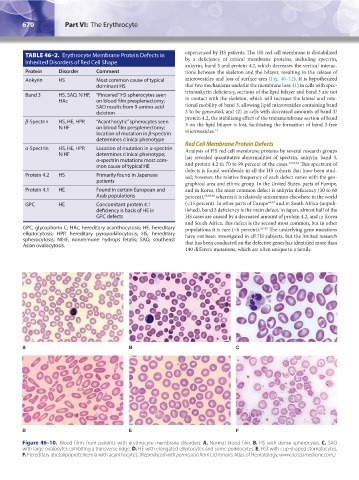
A B C
D E F
Figure 46–10. Blood films from patients with erythrocyte membrane disorders. A. Normal blood film. B. HS with dense spherocytes. C. SAO
with large ovalocytes exhibiting a transverse ridge. D. HE with elongated elliptocytes and some poikilocytes. E. HSt with cup-shaped stomatocytes.
F. Hereditary abetalipoproteinemia with acanthocytes. (Reproduced with permission from Lichtman’s Atlas of Hematology, www.accessmedicine.com.)
Kaushansky_chapter 46_p0661-0688.indd 670 9/17/15 6:42 PM