Page 222 - Clinical Immunology_ Principles and Practice ( PDFDrive )
P. 222
CHaPTEr 13 Regulated Necrosis and Its Immunogenicity 201
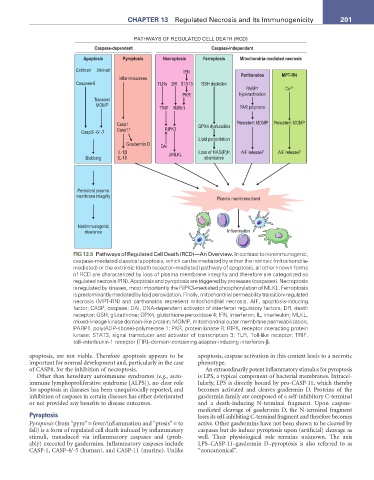
PATHWAYS OF REGULATED CELL DEATH (RCD)
Caspase-dependent Caspase-independent
Apoptosis Pyroptosis Necroptosis Ferroptosis Mitochondria-mediated necrosis
Extrinsic Intrinsic IFN
Inflammasomes Parthanatos MPT-RN
Caspase-8 TLRs DR STAT3 GSH depletion
PARP1 Ca 2+
PKR hyperactivation
Transient
MOMP
TRIF RIPK1 PAR polymers
Casp1 GPX4 dysfunction Persistent MOMP Persistent MOMP
Casp11 RIPK3
Casp3/ -6/ -7
Lipid peroxidation
Gasdermin D DAI
IL-1β pMLKL Loss of NAD(P)H AIF release? AIF release?
Blebbing IL-18 abundance
Persistent plasma
membrane integrity Plasma membrane burst
Nonimmunogenic
clearance Inflammation
FIG 13.5 Pathways of Regulated Cell Death (RCD)—An Overview. In contrast to nonimmunogenic,
caspase-mediated classical apoptosis, which can be mediated by either the intrinsic (mitochondria-
mediated) or the extrinsic (death receptor–mediated) pathway of apoptosis, all other known forms
of RCD are characterized by loss of plasma membrane integrity and therefore are categorized as
regulated necrosis (RN). Apoptosis and pyroptosis are triggered by proteases (caspases). Necroptosis
is regulated by kinases, most importantly the RIPK3-mediated phosphorylation of MLKL. Ferroptosis
is predominantly mediated by lipid peroxidation. Finally, mitochondrial permeability transition- regulated
necrosis (MPT-RN) and parthanatos represent mitochondrial necrosis. AIF, apoptosis-inducing
factor; CASP, caspase; DAI, DNA-dependent activator of interferon regulatory factors; DR, death
receptor; GSH, glutathione; GPX4, glutathione peroxidase 4; IFN, interferon; IL, interleukin; MLKL,
mixed-lineage kinase domain-like protein; MOMP, mitochondrial outer membrane permeabilization;
PARP1, poly(ADP-ribose)-polymerase 1; PKR, protein kinase R; RIPK, receptor interacting protein
kinase; STAT3, signal transducer and activator of transcription 3; TLR, Toll-like receptor; TRIF,
toll–interleukin-1 receptor [TIR]–domain–containing adapter-inducing interferon-β.
apoptosis, are not viable. Therefore apoptosis appears to be apoptosis, caspase activation in this context leads to a necrotic
important for normal development and, particularly in the case phenotype.
of CASP8, for the inhibition of necroptosis. An extraordinarily potent inflammatory stimulus for pyroptosis
Other than hereditary autoimmune syndromes (e.g., auto- is LPS, a typical component of bacterial membranes. Intracel-
immune lymphoproliferative syndrome [ALPS]), no clear role lularly, LPS is directly bound by pro-CASP-11, which thereby
for apoptosis in diseases has been unequivocally reported, and becomes activated and cleaves gasdermin D. Proteins of the
inhibition of caspases in certain diseases has either deteriorated gasdermin family are composed of a self-inhibitory C-terminal
or not provided any benefits to disease outcomes. and a death-inducing N-terminal fragment. Upon caspase-
mediated cleavage of gasdermin D, the N-terminal fragment
Pyroptosis loses its self-inhibiting C-terminal fragment and therefore becomes
Pyroptosis (from “pyro” = fever/inflammation and “ptosis” = to active. Other gasdermins have not been shown to be cleaved by
fall) is a form of regulated cell death induced by inflammatory caspases but do induce pyroptosis upon (artificial) cleavage as
stimuli, transduced via inflammatory caspases and (prob- well. Their physiological role remains unknown. The axis
ably) executed by gasdermins. Inflammatory caspases include LPS–CASP-11–gasdermin D–pyroptosis is also referred to as
CASP-1, CASP-4/-5 (human), and CASP-11 (murine). Unlike “noncanonical”.