Page 416 - Clinical Immunology_ Principles and Practice ( PDFDrive )
P. 416
CHAPtER 27 Host Defenses to Extracellular Bacteria 397
the MHC-like molecule CD1d. iNKT cells can be directly activated
by bacterial pathogens as a result of binding of cell wall com-
ponents of gram-negative bacteria to CD1d. Interestingly,
cytokines secreted by APCs that have encountered bacterial
pathogens can increase iNKT-cell accumulation of self-lipid
antigen for CD1d activation. Mucosa-associated invariant T
(MAIT) cells are restricted by MR1, a MHC-like receptor that
binds molecules derived from the microbial riboflavin synthesis
pathway. 24,26 Both gram-positive and gram-negative bacteria have
been shown to activate MAIT cells. Murine models of intraperi-
toneal inoculation of gram-negative bacteria suggest the MAIT
cells are important for the early clearance of pathogens. 25,26
Immunoglobulins
Immunoglobulins (Igs), principally secretory IgA and IgG, are
present at mucosal surfaces and in mucosal secretions. Important
in the generation of these immunoglobulins at mucosal surfaces
is the dissemination of IgA and IgG class–committed B- and
T-helper (Th) cells with specificity to an antigen encountered
and processed at one mucosal site to local and distant mucosal
sites. Protective mucosal antibodies against bacteria may be
derived from prior colonization, vaccines, or shared cross-reactive
antigens on normal flora. Mucosal Igs may neutralize bacterial
toxins, facilitate phagocytosis or bactericidal activity, inhibit
bacterial adherence ligands, or sterically hinder other events
necessary for bacterial colonization and invasion. Many extracel-
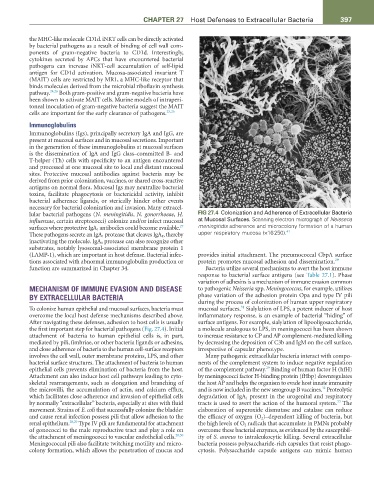
lular bacterial pathogens (N. meningitidis, N. gonorrhoeae, H. FIG 27.4 Colonization and Adherence of Extracellular Bacteria
influenzae, certain streptococci) colonize and/or infect mucosal at Mucosal Surfaces. Scanning electron micrograph of Neisseria
27
surfaces where protective IgA 1 antibodies could become available. meningitidis adherence and microcolony formation of a human
41
These pathogens secrete an IgA 1 protease that cleaves IgA 1 , thereby upper respiratory mucosa (×16 250).
inactivating the molecule. IgA 1 protease can also recognize other
substrates, notably lysosomal-associated membrane protein 1
(LAMP-1), which are important in host defense. Bacterial infec- provides initial attachment. The pneumococcal CbpA surface
tions associated with abnormal immunoglobulin production or protein promotes mucosal adhesion and dissemination. 29
function are summarized in Chapter 34. Bacteria utilize several mechanisms to avert the host immune
response to bacterial surface antigens (see Table 27.1). Phase
variation of adhesins is a mechanism of immune evasion common
MECHANISM OF IMMUNE EVASION AND DISEASE to pathogenic Neisseria spp. Meningococcus, for example, utilizes
BY EXTRACELLULAR BACTERIA phase variation of the adhesion protein Opa and type IV pili
during the process of colonization of human upper respiratory
31
To colonize human epithelial and mucosal surfaces, bacteria must mucosal surfaces. Sialylation of LPS, a potent inducer of host
overcome the local host defense mechanisms described above. inflammatory response, is an example of bacterial “hiding” of
After navigating these defenses, adhesion to host cells is usually surface antigens. For example, sialylation of lipooligosaccharide,
the first important step for bacterial pathogens (Fig. 27.4). Initial a molecule analogous to LPS, in meningococci has been shown
attachment of bacteria to human epithelial cells is, in part, to increase resistance to CP and AP complement-mediated killing
mediated by pili, fimbriae, or other bacteria ligands or adhesins, by decreasing the deposition of C3b and IgM on the cell surface,
and close adherence of bacteria to the human cell-surface receptors irrespective of capsular phenotype.
involves the cell wall, outer membrane proteins, LPS, and other Many pathogenic extracellular bacteria interact with compo-
bacterial surface structures. The attachment of bacteria to human nents of the complement system to induce negative regulation
29
epithelial cells prevents elimination of bacteria from the host. of the complement pathway. Binding of human factor H (hfH)
Attachment can also induce host cell pathways leading to cyto- by meningococci factor H-binding protein (fHbp) downregulates
skeletal rearrangements, such as elongation and branching of the host AP and helps the organism to evade host innate immunity
32
the microvilli, the accumulation of actin, and calcium efflux, and is now included in the new serogroup B vaccines. Proteolytic
which facilitates close adherence and invasion of epithelial cells degradation of IgA 1 present in the urogenital and respiratory
33
by normally “extracellular” bacteria, especially at sites with fluid tracts is used to avert the action of the humoral system. The
movement. Strains of E. coli that successfully colonize the bladder elaboration of superoxide dismutase and catalase can reduce
and cause renal infection possess pili that allow adhesion to the the efficacy of oxygen (O 2 )–dependent killing of bacteria, but
renal epithelium. 28,29 Type IV pili are fundamental for attachment the high levels of O 2 radicals that accumulate in PMNs probably
of gonococci to the male reproductive tract and play a role on overcome these bacterial enzymes, as evidenced by the susceptibil-
the attachment of meningococci to vascular endothelial cells. 28,30 ity of S. aureus to intraleukocytic killing. Several extracellular
Meningococcal pili also facilitate twitching motility and micro- bacteria possess polysaccharide-rich capsules that resist phago-
colony formation, which allows the penetration of mucus and cytosis. Polysaccharide capsule antigens can mimic human