Page 59 - Clinical Immunology_ Principles and Practice ( PDFDrive )
P. 59
44 ParT ONE Principles of Immune Response
and represent the majority of NK cells in peripheral lymphoid sites for the activation of other protein tyrosine kinases, such as
organs. NK cells are a major source of IFN-γ, which augments Syk and ZAP-70, which activate downstream effector molecules
the microbicidal functions of macrophages. Conversely, NK cells in a signaling cascade. Infection of host cells with some viruses
are primed by IL-15 derived from DCs and IL-12 or IL-18 derived can lead to reduced MHC class I expression, thereby reducing
from macrophages, demonstrating the regulatory interactions viral antigen presentation to T cells. Concomitantly, ligands for
that occur between NK cells and other cells of the immune activating receptors are expressed by the infected cell, leading
system. to NK-cell activation and killing of the infected cell.
NK-cell function is regulated by a delicate balance between NK cells play an important role in immunosurveillance against
32
33
signals generated by inhibitory and activating receptors. NK tumors. In humans, NK cell receptors that mediate tumor
cells possess the ability to recognize and kill infected or malig- recognition include NKp46, NKp30, NKp44, DNAM-1 (DNAX
nantly transformed cells, while leaving healthy host cells accessory molecule-1), and NKG2D. Ligands expressed on target
unharmed. Inhibitory receptors on NK cells recognize class I cells include MHC I-related chain (MIC)-A, MICB, unique long
major histocompatibility complex (MHC) molecules expressed 16-binding proteins (ULBP), poliovirus receptor (PVR), and
on most healthy cells in the body. Nectin-2. DNAM-1 specific ligands include PVR and Nectin-2,
NK inhibitory receptors include three families of receptors: which are expressed in cell lines that include carcinomas, mela-
heterodimers composed of CD94 and NKG2A, the Ig-like nomas, and neuroblastomas. Nectin expression is not specific
transcripts (i.e., ILT-2), and the killer cell Ig-like receptor (KIR) to tumors, since nectins are expressed on normal cells. DNAM-
family. The immunoreceptor tyrosine-based inhibition motifs 1-nectin interactions on normal cells do not result in NK cell
(ITIMs) in their cytoplasmic tails recruit phosphatases (Src lysis because normal cells are protected by MHC class I expression.
homology region 2 [SH2] domain-containing phosphatase-1 Conditions favoring NK cell–mediated lysis include tumors in
[SHP-1], SHP-2, and SHIP [SH2-containing inositol polyphos- which nectins are overexpressed and/or MHC class I expression
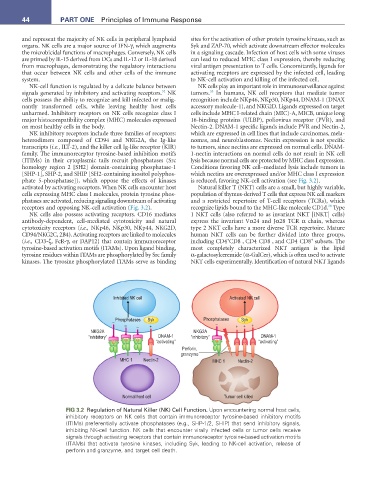
phate 5-phosphatase]), which oppose the effects of kinases is reduced, favoring NK-cell activation (see Fig. 3.2).
activated by activating receptors. When NK cells encounter host Natural killer T (NKT) cells are a small, but highly variable,
cells expressing MHC class I molecules, protein tyrosine phos- population of thymus-derived T cells that express NK cell markers
phatases are activated, reducing signaling downstream of activating and a restricted repertoire of T-cell receptors (TCRs), which
34
receptors and opposing NK-cell activation (Fig. 3.2). recognize lipids bound to the MHC-like molecule CD1d. Type
NK cells also possess activating receptors. CD16 mediates 1 NKT cells (also referred to as invariant NKT [iNKT] cells)
antibody-dependent, cell-mediated cytotoxicity and natural express the invariant Vα24 and Jα28 TCR α chain, whereas
cytotoxicity receptors (i.e., NKp46, NKp30, NKp44, NKG2D, type 2 NKT cells have a more diverse TCR repertoire. Mature
CD94/NKG2C, 2B4). Activating receptors are linked to molecules human NKT cells can be further divided into three groups,
−
−
+
+
−
−
(i.e., CD3-ζ, FcR-γ, or DAP12) that contain immunoreceptor including CD4 CD8 , CD4 CD8 , and CD4 CD8 subsets. The
tyrosine-based activation motifs (ITAMs). Upon ligand binding, most completely characterized NKT antigen is the lipid
tyrosine residues within ITAMs are phosphorylated by Src family α-galactosylceremide (α-GalCer), which is often used to activate
kinases. The tyrosine phosphorylated ITAMs serve as binding NKT cells experimentally. Identification of natural NKT ligands
Inhibited NK cell Activated NK cell
Phosphatases Syk Phosphatases Syk
–
– – + – + + +
NKG2A NKG2A
“inhibitory” DNAM-1 “inhibitory” DNAM-1
“activating” “activating”
Perforin,
granzyme
MHC 1 Nectin-2 MHC 1 Nectin-2
Normal host cell Tumor cell killed
FIG 3.2 Regulation of Natural Killer (NK) Cell Function. Upon encountering normal host cells,
inhibitory receptors on NK cells that contain immunoreceptor tyrosine-based inhibitory motifs
(ITIMs) preferentially activate phosphatases (e.g., SHP-1/2, SHIP) that send inhibitory signals,
inhibiting NK-cell function. NK cells that encounter virally infected cells or tumor cells receive
signals through activating receptors that contain immunoreceptor tyrosine-based activation motifs
(ITAMs) that activate tyrosine kinases, including Syk, leading to NK-cell activation, release of
perforin and granzyme, and target cell death.