Page 64 - Clinical Immunology_ Principles and Practice ( PDFDrive )
P. 64
CHaPTEr 3 Innate Immunity 49
TLR4
TLR5
MAL TRAM TLR3
MyD88 Endosomes
TRIF TLR7,8,9
MyD88 Early
IRAK4 Late UNC93B
IRAK1 IRAK2 UNC93B
TRAF3 TRAF3 TRIF
RIP1 MyD88 TRAF3
IRF5 TRAF6 TRAF6 BTK OPNi
TAK1 MAPKs IRAK4 IRAK1 IKKα
TAK1 MAPKs
TBK1/IKKι TBK1/IKKι
IRF5 TRAF6
IKK TAK1 MAPKs IRF7
IKK IKK
AP1 IRF3
IκB IκB IκB AP1
NF-κB
NF-κB NF-κB
Nucleus Proinflammatory Type 1 Proinflammatory Type 1
cytokines interferons cytokines interferons
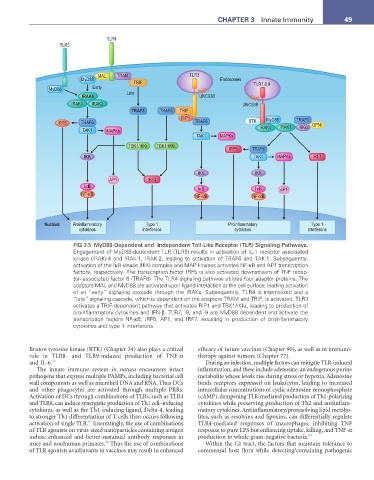
FIG 3.5 MyD88-Dependent and -Independent Toll-Like Receptor (TLR) Signaling Pathways.
Engagement of MyD88-dependent TLR (TLR5) results in activation of IL-1 receptor associated
kinase (IRAK)-4 and IRAK-1, IRAK-2, leading to activation of TRAF6 and TAK-1. Subsequently,
activation of the IκB kinase (IKK) complex and MAP kinases activates NF-κB and AP1 transcription
factors, respectively. The transcription factor IRF5 is also activated downstream of TNF recep-
tor–associated factor 6 (TRAF6). The TLR4 signaling pathway utilizes four adaptor proteins. The
adaptors MAL and MyD88 are activated upon ligand interaction at the cell surface, leading activation
of an “early” signaling cascade through the IRAKs. Subsequently, TLR4 is internalized and a
“late” signaling cascade, which is dependent on the adaptors TRAM and TRIF, is activated. TLR3
activates a TRIF-dependent pathway that activates RIP1 and TBK1/IKKι, leading to production of
proinflammatory cytokines and IFN-β. TLR7, -8, and -9 are MyD88 dependent and activate the
transcription factors NF-κB, IRF5, AP1, and IRF7, resulting in production of proinflammatory
cytokines and type 1 interferons.
Bruton tyrosine kinase (BTK) (Chapter 34) also plays a critical efficacy of future vaccines (Chapter 90), as well as in immuno-
role in TLR8- and TLR9-induced production of TNF-α therapy against tumors (Chapter 77).
and IL-6. 56 During an infection, multiple factors can mitigate TLR-induced
The innate immune system in natura encounters intact inflammation, and these include adenosine, an endogenous purine
pathogens that express multiple PAMPs, including bacterial cell metabolite whose levels rise during stress or hypoxia. Adenosine
wall components as well as microbial DNA and RNA. Thus DCs binds receptors expressed on leukocytes, leading to increased
and other phagocytes are activated through multiple PRRs. intracellular concentrations of cyclic adenosine monophosphate
Activation of DCs through combinations of TLRs, such as TLR4 (cAMP), dampening TLR-mediated production of Th1-polarizing
and TLR8, can induce synergistic production of Th1 cell–inducing cytokines while preserving production of Th2 and antiinflam-
cytokines, as well as the Th1-inducing ligand, Delta-4, leading matory cytokines. Antiinflammatory/proresolving lipid metabo-
to stronger Th1 differentiation of T cells than occurs following lites, such as resolvins and lipoxins, can differentially regulate
57
activation of single TLR. Interestingly, the use of combinations TLR4-mediated responses of macrophages, inhibiting TNF
of TLR agonists on virus-sized nanoparticles containing antigen response to pure LPS but enhancing uptake, killing, and TNF-α
induce enhanced and better-sustained antibody responses in production to whole gram-negative bacteria. 59
58
mice and nonhuman primates. Thus the use of combinations Within the GI tract, the factors that maintain tolerance to
of TLR agonists as adjuvants in vaccines may result in enhanced commensal host flora while detecting/containing pathogenic