Page 686 - Clinical Immunology_ Principles and Practice ( PDFDrive )
P. 686
CHaPTEr 48 Drug Hypersensitivity 659
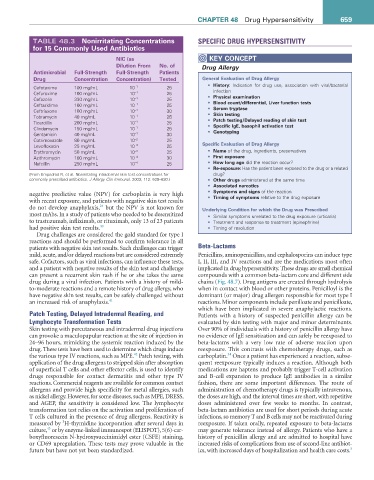
TABLE 48.3 Nonirritating Concentrations SPECIFIC DRUG HYPERSENSITIVITY
for 15 Commonly Used antibiotics
NiC (as KEY CONCEPT
Dilution From No. of Drug Allergy
antimicrobial Full-Strength Full-Strength Patients
Drug Concentration Concentration) Tested General Evaluation of Drug allergy
Cefotaxime 100 mg/mL 10 −1 25 • History: Indication for drug use, association with viral/bacterial
infection
Cefuroxime 100 mg/mL 10 −1 25 • Physical examination
Cefazolin 330 mg/mL 10 −1 25
Ceftazidime 100 mg/mL 10 −1 25 • Blood count/differential, Liver function tests
• Serum tryptase
Ceftriaxone 100 mg/mL 10 −1 30
Tobramycin 40 mg/mL 10 −1 25 • Skin testing
• Patch testing/Delayed reading of skin test
Ticarcillin 200 mg/mL 10 −1 25
Clindamycin 150 mg/mL 10 −1 25 • Specific igE, basophil activation test
• Genotyping
Gentamicin 40 mg/mL 10 −1 30
Cotrimoxazole 80 mg/mL 10 −2 25
Levofloxacin 25 mg/mL 10 −3 25 Specific Evaluation of Drug allergy
Erythromycin 50 mg/mL 10 −3 25 • Name of the drug, ingredients, preservatives
Azithromycin 100 mg/mL 10 −4 30 • First exposure
Nafcillin 250 mg/mL 10 −4 25 • How long ago did the reaction occur?
• re-exposure: Has the patient been exposed to the drug or a related
(From Empedrad R, et al. Nonirritating intradermal skin test concentrations for drug?
commonly prescribed antibiotics. J Allergy Clin Immunol. 2003; 112: 629–630.) • Other drugs administered at the same time
• associated narcotics
• Symptoms and signs of the reaction
negative predictive value (NPV) for carboplatin is very high • Timing of symptoms relative to the drug exposure
with recent exposure, and patients with negative skin test results
24
do not develop anaphylaxis, but the NPV is not known for Underlying Condition for which the Drug was Prescribed
most mAbs. In a study of patients who needed to be desensitized • Similar symptoms unrelated to the drug exposure (urticaria)
to trastuzumab, infliximab, or rituximab, only 13 of 23 patients • Treatment and response to treatment (epinephrine)
had positive skin test results. 40 • Timing of resolution
Drug challenges are considered the gold standard for type I
reactions and should be performed to confirm tolerance in all
patients with negative skin test results. Such challenges can trigger Beta-Lactams
mild, acute, and/or delayed reactions but are considered extremely Penicillins, aminopenicillins, and cephalosporins can induce type
safe. Cofactors, such as viral infections, can influence these tests, I, II, III, and IV reactions and are the medications most often
and a patient with negative results of the skin test and challenge implicated in drug hypersensitivity. These drugs are small chemical
can present a recurrent skin rash if he or she takes the same compounds with a common beta-lactam core and different side
drug during a viral infection. Patients with a history of mild- chains (Fig. 48.7). Drug antigens are created through hydrolysis
to-moderate reactions and a remote history of drug allergy, who when in contact with blood or other proteins. Penicilloyl is the
have negative skin test results, can be safely challenged without dominant (or major) drug allergen responsible for most type I
an increased risk of anaphylaxis. 41 reactions. Minor components include penilloate and penicilloate,
which have been implicated in severe anaphylactic reactions.
Patch Testing, Delayed Intradermal Reading, and Patients with a history of suspected penicillin allergy can be
Lymphocyte Transformation Tests evaluated by skin testing with major and minor determinants.
Skin testing with percutaneous and intradermal drug injections Over 90% of individuals with a history of penicillin allergy have
can provoke a maculopapular reaction at the site of injection in no evidence of IgE sensitization and can safely be reexposed to
24–96 hours, mimicking the systemic reaction induced by the beta-lactams with a very low rate of adverse reaction upon
drug. These tests have been used to determine which drugs induce reexposure. This contrasts with chemotherapy drugs, such as
34
42
the various type IV reactions, such as MPE. Patch testing, with carboplatin. Once a patient has experienced a reaction, subse-
application of the drug allergens to stripped skin after absorption quent reexposure typically induces a reaction. Although both
of superficial T cells and other effector cells, is used to identify medications are haptens and probably trigger T-cell activation
drugs responsible for contact dermatitis and other type IV and B-cell expansion to produce IgE antibodies in a similar
reactions. Commercial reagents are available for common contact fashion, there are some important differences. The route of
allergens and provide high specificity for metal allergies, such administration of chemotherapy drugs is typically intravenous,
as nickel allergy. However, for some diseases, such as MPE, DRESS, the doses are high, and the interval times are short, with repetitive
and AGEP, the sensitivity is considered low. The lymphocyte doses administered over few weeks to months. In contrast,
transformation test relies on the activation and proliferation of beta-lactam antibiotics are used for short periods during acute
T cells cultured in the presence of drug allergens. Reactivity is infections, so memory T and B cells may not be reactivated during
3
measured by H-thymidine incorporation after several days in reexposure. If taken orally, repeated exposure to beta-lactams
43
culture, or by enzyme-linked immunospot (ELISPOT), 5(6)-car- may generate tolerance instead of allergy. Patients who have a
boxyfluorescein N-hydroxysuccinimidyl ester (CSFE) staining, history of penicillin allergy and are admitted to hospital have
or CD69 upregulation. These tests may prove valuable in the increased risks of complications from use of second-line antibiot-
5
future but have not yet been standardized. ics, with increased days of hospitalization and health care costs.