Page 848 - Clinical Immunology_ Principles and Practice ( PDFDrive )
P. 848
820 Part SIX Systemic Immune Diseases
Histomorphological reports describe mononuclear cell infiltrates. Marked wall thickening with inflammatory tissue
infiltrates penetrating through all layers of the vessel wall (Fig. extending into perivascular structures is typical for TA (Fig.
1
59.9). Recent discussions have focused on the diagnostic relevance 59.10). Destruction of elastic membranes is often extensive and
of isolated inflammatory cell clusters in the adventitia or peri- combined with patchy areas of media necrosis. Weakening of
36
vascular lymphocytes limited to small blood vessels. These the vessel wall can lead to aneurysm formation. Inflammatory
findings may not be sufficient to indicate arteritis. Multinucleated lesions may be arranged in a “skipped” pattern, with normal
giant cells may or may not be found. They tend to lie along the vessel wall segments alternating with stretches of intense destruc-
internal elastic lamina, at the junction between the media and tive inflammation.
the intima. Media destruction is not unusual, but findings of Physicians may encounter morphological findings of granu-
fibrinoid necrosis should prompt a search for a different vasculitic lomatous aortitis in patients undergoing aortic aneurysm repair
entity. The vessel lumen is more or less compromised by hyper- without any prior diagnosis of vasculitis. Detailed workup of
plastic intima formed from proliferating fibroblasts, smooth- these patients is necessary to identify those with undiagnosed
muscle cells, and deposition of acid mucopolysaccharides. PMR, GCA, or TA. Rare causes of aortitis, including inflammatory
The histology of TA is similar to that of GCA, making it bowel disease, sarcoidosis, syphilis, relapsing polychondritis, and
difficult to dissect both syndromes in tissue samples derived connective tissue disease, should be ruled out. Isolated granu-
from the aorta or its primary branches. Lymphocytes and plasma lomatous aortitis is diagnosed as idiopathic aortitis. The
cells accumulate around vasa vasorum and form transmural pathogenesis and prognosis of this condition are essentially
unknown.
Diagnostic Imaging
TABLE 59.5 american College of Modern imaging modalities have fundamentally changed the
26
a
rheumatology 1990 Criteria for the diagnostic approach to LVV. Indeed, diagnosing TA mostly
Classification of takayasu arteritis depends on identifying vascular lesions in typical distribution
by imaging. 37
Disease onset at ≤40 years Conventional angiography still has its place in preoperative
Claudication of an extremity planning and can be combined with intravascular interventions.
Decreased brachial artery pulse
>10 mm Hg difference in systolic blood pressure between arms It provides ideal visualization of the vascular lumen not only
Bruit over the subclavian arteries or the aorta for large but also for medium-sized arteries, such as the axillary
Arteriographic evidence of narrowing or occlusion of the entire aorta, and brachial arteries (see Fig. 59.5). Ultrasound (US)–based
its primary branches, or large arteries in the proximal upper or lower methods are extremely useful for screening carotid arteries, but
extremities they have also emerged as the method of choice for initial assess-
ment of the distal subclavian arteries, vertebral arteries, renal
a For purposes of classification, a patient is classified as having Takayasu arteritis if
more than three of the six criteria are fulfilled. arteries, and femoral arteries. US examination is also the optimal
Reprinted from Arend WP, Michel BA, Bloch DA, et al. The American College of method for long-term monitoring of vessel bypasses in patients
Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum who have undergone revascularization surgical procedures. Magnetic
1990; 33: 1129–1134, with permission of Wiley-Liss, Inc., a subsidiary of John Wiley
& Sons, Inc. ©1990. resonance imaging (MRI), magnetic resonance angiography (MRA),
$ %
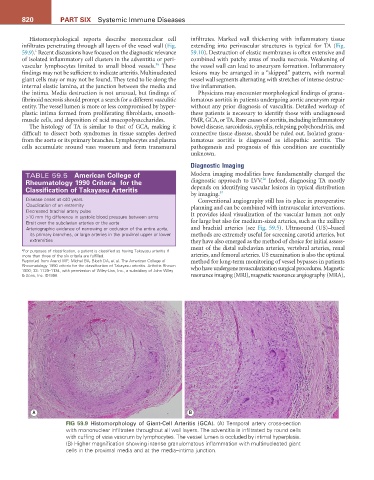
FIG 59.9 Histomorphology of Giant-Cell Arteritis (GCA). (A) Temporal artery cross-section
with mononuclear infiltrates throughout all wall layers. The adventitia is infiltrated by round cells
with cuffing of vasa vasorum by lymphocytes. The vessel lumen is occluded by intimal hyperplasia.
(B) Higher magnification showing intense granulomatous inflammation with multinucleated giant
cells in the proximal media and at the media–intima junction.