Page 86 - Clinical Immunology_ Principles and Practice ( PDFDrive )
P. 86
CHaPter 4 Antigen Receptor Genes, Gene Products, and Coreceptors 71
Ag
CD22
IgG
BCR
BCR
Ig-α FcRγIIB Ig-α
Ig-β Ig-β
PTK Lyn
Syk Syk
–
SHIP or – SHP-1
SHP-1
A B
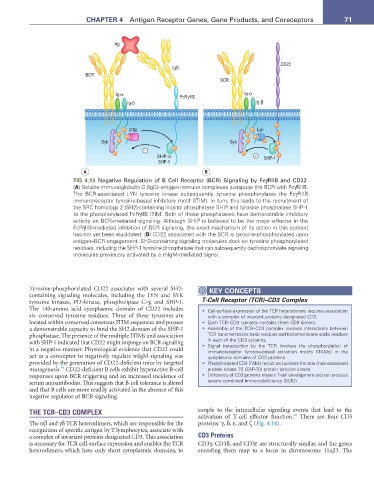
FIG 4.13 Negative Regulation of B Cell Receptor (BCR) Signaling by FcγRIIB and CD22.
(a) Soluble immunoglobulin G (IgG)–antigen immune complexes juxtapose the BCR with FcγRIIB.
The BCR-associated LYN tyrosine kinase subsequently tyrosine phosphorylates the FcγRIIB
immunoreceptor tyrosine-based inhibitory motif (ITIM). In turn, this leads to the recruitment of
the SRC homology 2 (SH2)-containing inositol phosphatase SHIP and tyrosine phosphatase SHP-1
to the phosphorylated FcRγIIB ITIM. Both of these phosphatases have demonstrable inhibitory
activity on BCR-mediated signaling. Although SHIP is believed to be the major effector in the
FcRγIIB-mediated inhibition of BCR signaling, the exact mechanism of its action in this context
has not yet been elucidated. (B) CD22 associated with the BCR is tyrosine-phosphorylated upon
antigen–BCR engagement. SH2-containing signaling molecules dock on tyrosine phosphorylated
residues, including the SHP-1 tyrosine phosphatase that can subsequently dephosphorylate signaling
molecules previously activated by a mIgM-mediated signal.
Tyrosine-phosphorylated CD22 associates with several SH2- KeY ConCePtS
containing signaling molecules, including the LYN and SYK
tyrosine kinases, PI3-kinase, phospholipase C-γ, and SHP-1. T-Cell Receptor (TCR)–CD3 Complex
The 140-amino acid cytoplasmic domain of CD22 includes • Cell-surface expression of the TCR heterodimers requires association
six conserved tyrosine residues. Three of these tyrosines are with a complex of invariant proteins designated CD3.
located within conserved consensus ITIM sequences and possess • Each TCR–CD3 complex contains three CD3 dimers.
a demonstrable capacity to bind the SH2 domain of the SHP-1 • Assembly of the TCR–CD3 complex involves interactions between
phosphatase. The presence of the multiple ITIMs and association TCR transmembrane basic residues and transmembrane acidic residues
with SHP-1 indicated that CD22 might impinge on BCR signaling in each of the CD3 subunits.
in a negative manner. Physiological evidence that CD22 could • Signal transduction by the TCR involves the phosphorylation of
immunoreceptor tyrosine-based activation motifs (ITAMs) in the
act as a coreceptor to negatively regulate mIgM signaling was cytoplasmic domains of CD3 proteins.
provided by the generation of CD22-deficient mice by targeted • Phosphorylated CD3 ITAMs recruit and activate the zeta chain-associated
59
mutagenesis. CD22-deficient B cells exhibit hyperactive B-cell protein kinase 70 (ZAP-70) protein tyrosine kinase.
responses upon BCR triggering and an increased incidence of • Deficiency of CD3 proteins impairs T-cell development and can produce
serum autoantibodies. This suggests that B-cell tolerance is altered severe combined immunodeficiency (SCID).
and that B cells are more readily activated in the absence of this
negative regulator of BCR signaling.
THE TCR–CD3 COMPLEX couple to the intracellular signaling events that lead to the
60
activation of T-cell effector function. There are four CD3
The αβ and γδ TCR heterodimers, which are responsible for the proteins: γ, δ, ε, and ζ (Fig. 4.14).
recognition of specific antigen by T lymphocytes, associate with
a complex of invariant proteins designated CD3. This association CD3 Proteins
is necessary for TCR cell-surface expression and enables the TCR CD3γ, CD3δ, and CD3ε are structurally similar, and the genes
heterodimers, which have only short cytoplasmic domains, to encoding them map to a locus in chromosome 11q23. The