Page 962 - Clinical Immunology_ Principles and Practice ( PDFDrive )
P. 962
CHaPter 68 Immunological Renal Diseases 929
Caucasians. Nephritis is a major cause of morbidity and mortality
and accounts for a large portion of all hospital admissions in
patients with SLE.
Pathogenesis
Several different mechanisms appear to be involved in the
pathogenesis of lupus nephritis, resulting in a wide spectrum of
renal lesions. Deposition of immune complexes from the circula-
tion into the kidney appears to be the initiating event in prolifera-
tive lupus nephritis; however, only a subset of immune complexes
appears to be nephritogenic. DNA and anti-DNA antibodies are
known to be concentrated in glomerular deposits in the suben-
dothelial location and are likely to play a central role in the
pathogenesis of proliferative lupus nephritis. Unfortunately, there
are fewer insights into the pathogenesis of lupus MN with its
characteristic epimembranous immune deposits. Although T
cells are almost certainly involved in autoantibody production,
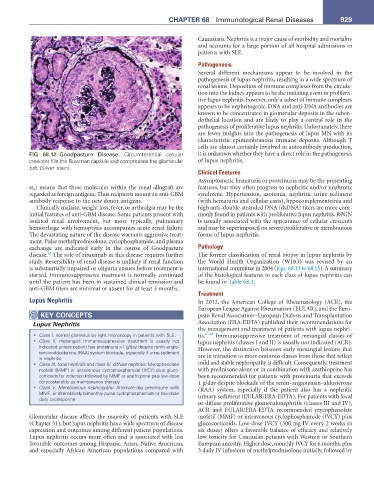
FIG 68.12 Goodpasture Disease. Circumferential cellular it is unknown whether they have a direct role in the pathogenesis
crescent fills the Bowman capsule and compresses the glomerular of lupus nephritis.
tuft (Silver stain).
Clinical Features
Asymptomatic hematuria or proteinuria may be the presenting
α 4 ) means that these molecules within the renal allograft are features, but they often progress to nephritic and/or nephrotic
regarded as foreign antigens. Thus recipients mount an anti-GBM syndrome. Hypertension, azotemia, nephritic urine sediment
antibody response to the new donor antigens. (with hematuria and cellular casts), hypocomplementemia and
Clinically malaise, weight loss, fever, or arthralgia may be the high anti–double-stranded DNA (dsDNA) titers are more com-
initial features of anti-GBM disease. Some patients present with monly found in patients with proliferative lupus nephritis. RPGN
isolated renal involvement, but more typically, pulmonary is usually associated with the appearance of cellular crescents
hemorrhage with hemoptysis accompanies acute renal failure. and may be superimposed on severe proliferative or membranous
The devastating nature of the disease warrants aggressive treat- forms of lupus nephritis.
ment. Pulse methylprednisolone, cyclophosphamide, and plasma
exchange are indicated early in the course of Goodpasture Pathology
42
disease. The role of rituximab in this disease requires further The former classification of renal biopsy in lupus nephritis by
study. Reversibility of renal disease is unlikely if renal function the World Health Organization (WHO) was revised by an
is substantially impaired or oliguria ensues before treatment is international committee in 2004 (Figs. 68.13 to 68.15). A summary
started. Immunosuppressive treatment is normally continued of the histological features in each class of lupus nephritis can
until the patient has been in sustained clinical remission and be found in Table 68.3.
anti-GBM titers are minimal or absent for at least 3 months.
Treatment
Lupus Nephritis In 2012, the American College of Rheumatology (ACR), the
European League Against Rheumatism (EULAR), and the Euro-
KeY COnCePtS pean Renal Association–European Dialysis and Transplantation
Lupus Nephritis Association (ERA-EDTA) published their recommendations for
the management and treatment of patients with lupus nephri-
• Class I: normal glomeruli by light microscopy in patients with SLE. tis. 43,44 Immunosuppressive treatment of mesangial classes of
• Class II, mesangial: Immunosuppressive treatment is usually not lupus nephritis (classes I and II) is usually not indicated (ACR).
indicated unless patient has proteinuria >1 g/day despite renin–angio- However, the distinction between early mesangial lesions that
tensin–aldosterone (RAA) system blockade, especially if urine sediment
is nephritic are in transition to more ominous classes from those that reflect
• Class III, focal nephritis and class IV, diffuse nephritis: Mycophenolate mild and stable nephropathy is difficult. Consequently, treatment
mofetil (MMF) or intravenous cyclophosphamide (IVCY) plus gluco- with prednisone alone or in combination with azathioprine has
corticoids for induction followed by MMF or azathioprine plus low-dose been recommended for patients with proteinuria that exceeds
corticosteroids as maintenance therapy 1 g/day despite blockade of the renin–angiotensin–aldosterone
• Class V, Membranous nephropathy: Alternate-day prednisone with (RAA) system, especially if the patient also has a nephritic
MMF, or alternatively bimonthly pulse cyclophosphamide or low-dose urinary sediment (EULAR/ERA-EDTA). For patients with focal
daily cyclosporine
or diffuse proliferative glomerulonephritis (classes III and IV),
ACR and EULAR/ERA-EDTA recommended mycophenolate
Glomerular disease affects the majority of patients with SLE mofetil (MMF) or intravenous cyclophosphamide (IVCY) plus
(Chapter 51), but lupus nephritis has a wide spectrum of disease glucocorticoids. Low-dose IVCY (500 mg IV every 2 weeks in
expression and outcomes among different patient populations. six doses) offers a favorable balance of efficacy and relatively
Lupus nephritis occurs more often and is associated with less low toxicity for Caucasian patients with Western or Southern
favorable outcomes among Hispanic, Asian, Native American, European ancestry. Higher dose, monthly IVCY for 6 months, plus
and especially African American populations compared with 3 daily IV infusions of methylprednisolone initially, followed by