Page 111 - 9780077418427.pdf
P. 111
/Users/user-f465/Desktop
tiL12214_ch04_085-114.indd Page 88 9/1/10 9:37 PM user-f465
tiL12214_ch04_085-114.indd Page 88 9/1/10 9:37 PM user-f465 /Users/user-f465/Desktop
molecular theory, molecules of ammonia leave the bottle and
TABLE 4.1
bounce around among the other molecules making up the air
The shape and volume characteristics of solids, liquids, and until they are everywhere in the room, slowly becoming more
gases are reflections of their molecular arrangements*
evenly distributed. The ammonia molecules diff use, or spread,
Solids Liquids Gases throughout the room. The ammonia odor diff uses throughout
the room faster if the air temperature is higher and slower if
Shape Fixed Variable Variable the air temperature is lower. This would imply a relationship
Volume Fixed Fixed Variable between the temperature and the speed at which molecules
move about.
*These characteristics are what would be expected under ordinary temperature and The relationship between the temperature of a gas and
pressure conditions on the surface of Earth.
the motion of molecules was formulated in 1857 by Rudolf
Clausius. He showed that the temperature of a gas is pro-
portional to the average kinetic energy of the gas molecules.
equilibrium position. The masses of these molecules and the This means that ammonia molecules have a greater average
spacing between them determine the density of the solid. Th e velocity at a higher temperature and a slower average veloc-
hardness of a solid is the resistance of a solid to forces that tend ity at a lower temperature. This explains why gases diffuse at
to push its molecules farther apart. a greater rate at higher temperatures. Recall, however, that
Liquids have molecules that are not confined to an equilib- kinetic energy involves the mass of the molecules as well as
2
rium position as in a solid. The molecules of a liquid are close their velocity (KE = 1/2 mv ). It is the average kinetic energy
together and bound by cohesive forces that are not as strong that is proportional to the temperature, which involves the
as in a solid. This permits the molecules to move from place to molecular mass as well as the molecular velocity. Whether
place within the liquid. The molecular forces are strong enough the kinetic energy is jiggling, vibrating, rotating, or moving
to give the liquid a definite volume but not strong enough to from place to place, the temperature of a substance is a mea-
give it a definite shape. Thus, a liter of water is always a liter of sure of the average kinetic energy of the molecules making up
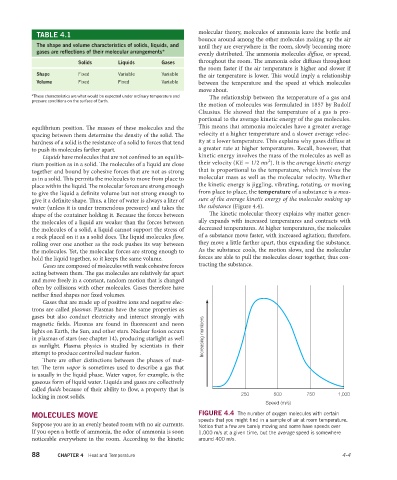
water (unless it is under tremendous pressure) and takes the the substance (Figure 4.4).
shape of the container holding it. Because the forces between The kinetic molecular theory explains why matter gener-
the molecules of a liquid are weaker than the forces between ally expands with increased temperatures and contracts with
the molecules of a solid, a liquid cannot support the stress of decreased temperatures. At higher temperatures, the molecules
a rock placed on it as a solid does. The liquid molecules fl ow, of a substance move faster, with increased agitation; therefore,
rolling over one another as the rock pushes its way between they move a little farther apart, thus expanding the substance.
the molecules. Yet, the molecular forces are strong enough to As the substance cools, the motion slows, and the molecular
hold the liquid together, so it keeps the same volume. forces are able to pull the molecules closer together, thus con-
Gases are composed of molecules with weak cohesive forces tracting the substance.
acting between them. The gas molecules are relatively far apart
and move freely in a constant, random motion that is changed
often by collisions with other molecules. Gases therefore have
neither fixed shapes nor fi xed volumes.
Gases that are made up of positive ions and negative elec-
trons are called plasmas. Plasmas have the same properties as
gases but also conduct electricity and interact strongly with
magnetic fields. Plasmas are found in fluorescent and neon
lights on Earth, the Sun, and other stars. Nuclear fusion occurs
in plasmas of stars (see chapter 14), producing starlight as well Increasing numbers
as sunlight. Plasma physics is studied by scientists in their
attempt to produce controlled nuclear fusion.
There are other distinctions between the phases of mat-
ter. Th e term vapor is sometimes used to describe a gas that
is usually in the liquid phase. Water vapor, for example, is the
gaseous form of liquid water. Liquids and gases are collectively
called fl uids because of their ability to flow, a property that is
lacking in most solids. 250 500 750 1,000
Speed (m/s)
MOLECULES MOVE FIGURE 4.4 The number of oxygen molecules with certain
speeds that you might find in a sample of air at room temperature.
Suppose you are in an evenly heated room with no air currents. Notice that a few are barely moving and some have speeds over
If you open a bottle of ammonia, the odor of ammonia is soon 1,000 m/s at a given time, but the average speed is somewhere
noticeable everywhere in the room. According to the kinetic around 400 m/s.
88 CHAPTER 4 Heat and Temperature 4-4