Page 112 - 9780077418427.pdf
P. 112
/Users/user-f465/Desktop
tiL12214_ch04_085-114.indd Page 89 9/1/10 9:37 PM user-f465
tiL12214_ch04_085-114.indd Page 89 9/1/10 9:37 PM user-f465 /Users/user-f465/Desktop
CONCEPTS Applied Room temperature
Iron
Moving Molecules
Blow up a small balloon and make a knot in the neck so
it will not leak air. Note the size of the balloon. Place the
balloon in the freezer part of a refrigerator for an hour, Brass
then again note the size of the balloon. Immediately place A
the balloon in direct sunlight for an hour and again note the
size of the balloon. Explain your observations by using the When heated
kinetic molecular theory.
Iron
Brass
4.2 TEMPERATURE
If you ask people about the temperature, they usually respond
with a referent (“hotter than the summer of ’89”) or a number
(“68°F or 20°C”). Your response, or feeling, about the referent B
or number depends on a number of factors, including a rela-
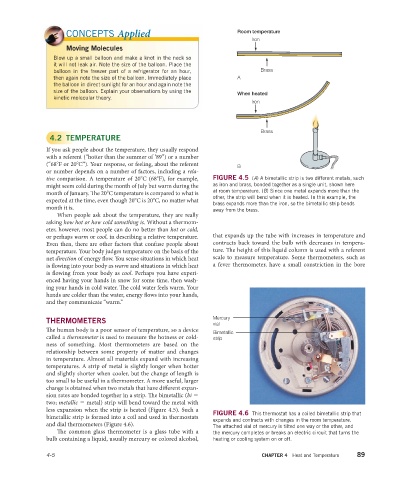
tive comparison. A temperature of 20°C (68°F), for example, FIGURE 4.5 (A) A bimetallic strip is two different metals, such
might seem cold during the month of July but warm during the as iron and brass, bonded together as a single unit, shown here
month of January. Th e 20°C temperature is compared to what is at room temperature. (B) Since one metal expands more than the
other, the strip will bend when it is heated. In this example, the
expected at the time, even though 20°C is 20°C, no matter what
brass expands more than the iron, so the bimetallic strip bends
month it is.
away from the brass.
When people ask about the temperature, they are really
asking how hot or how cold something is. Without a thermom-
eter, however, most people can do no better than hot or cold,
or perhaps warm or cool, in describing a relative temperature. that expands up the tube with increases in temperature and
Even then, there are other factors that confuse people about contracts back toward the bulb with decreases in tempera-
temperature. Your body judges temperature on the basis of the ture. The height of this liquid column is used with a referent
net direction of energy flow. You sense situations in which heat scale to measure temperature. Some thermometers, such as
is flowing into your body as warm and situations in which heat a fever thermometer, have a small constriction in the bore
is flowing from your body as cool. Perhaps you have experi-
enced having your hands in snow for some time, then wash-
ing your hands in cold water. The cold water feels warm. Your
hands are colder than the water, energy flows into your hands,
and they communicate “warm.”
THERMOMETERS Mercury
vial
The human body is a poor sensor of temperature, so a device
Bimetallic
called a thermometer is used to measure the hotness or cold- strip
ness of something. Most thermometers are based on the
relationship between some property of matter and changes
in temperature. Almost all materials expand with increasing
temperatures. A strip of metal is slightly longer when hotter
and slightly shorter when cooler, but the change of length is
too small to be useful in a thermometer. A more useful, larger
change is obtained when two metals that have diff erent expan-
sion rates are bonded together in a strip. The bimetallic (bi =
two; metallic = metal) strip will bend toward the metal with
less expansion when the strip is heated (Figure 4.5). Such a
FIGURE 4.6 This thermostat has a coiled bimetallic strip that
bimetallic strip is formed into a coil and used in thermostats
expands and contracts with changes in the room temperature.
and dial thermometers (Figure 4.6). The attached vial of mercury is tilted one way or the other, and
The common glass thermometer is a glass tube with a the mercury completes or breaks an electric circuit that turns the
bulb containing a liquid, usually mercury or colored alcohol, heating or cooling system on or off.
4-5 CHAPTER 4 Heat and Temperature 89