Page 240 - 9780077418427.pdf
P. 240
/Users/user-f465/Desktop
tiL12214_ch08_203-228.indd Page 217 9/1/10 9:45 PM user-f465
tiL12214_ch08_203-228.indd Page 217 9/1/10 9:45 PM user-f465 /Users/user-f465/Desktop
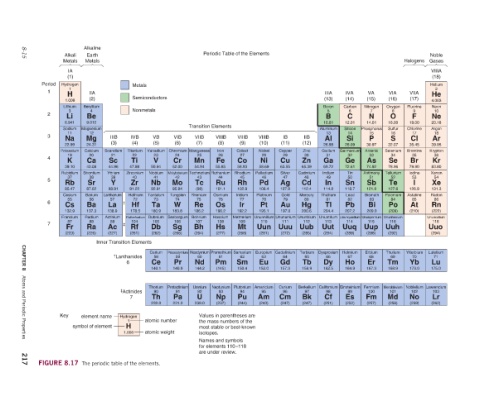
Noble Gases VIIIA (18) Helium 2 He 4.003 Neon 10 Ne 20.18 Argon 18 Ar 39.95 Krypton 36 Kr 83.80 Xenon 54 Xe 131.3 Radon 86 Rn (222) Ununoxtium 118 Uuo (294) Lutetium 71 Lu 175.0 Lawrencium 103 Lr (262)
Halogens VIIA (17) Fluorine 9 F 19.00 Chlorine 17 Cl 35.45 Bromine 35 Br 79.90 Iodine 53 I 126.9 Astatine 85 At (210) Ytterbium 70 Yb 173.0 Noblelium 102 No (259)
VIA (16) Oxygen 8 O 16.00 Sulfur 16 S 32.07 Selenium 34 Se 78.96 Tellurium 52 Te 127.6 Polonium 84 Po (209) Ununhexium 116 Uuh (292) Thulium 69 Tm 168.9 Mendelevium 101 Md (258)
VA (15) Nitrogen 7 N 14.01 Phosphorus 15 P 30.97 Arsenic 33 As 74.92 Antimony 51 Sb 121.8 Bismuth 83 Bi 209.0 Ununpentium 115 Uup (288) Erbium 68 Er 167.3 Fermium 100 Fm (257)
IVA (14) Carbon 6 C 12.01 Silicon 14 Si 28.09 Germanium 32 Ge 72.61 Tin 50 Sn 118.7 Lead 82 Pb 207.2 Ununquadium 114 Uuq (289) Holmium 67 Ho 164.9 Einsteinium 99 Es (252)
IIIA (13) Boron 5 B 10.81 Aluminum 13 Al 26.98 Gallium 31 Ga 69.72 Indium 49 In 114.8 Thallium 81 Tl 204.4 Ununtrium 113 Uut (284) Dysprosium 66 Dy 162.5 Californium 98 Cf (251)
IIB (12) Zinc 30 Zn 65.39 Cadmium 48 Cd 112.4 Mercury 80 Hg 200.6 Ununbium 112 Uub (285) Terbium 65 Tb 158.9 Berkelium 97 Bk (247)
IB VIIIB (11) (10) Copper Nickel 29 28 Cu Ni 63.55 58.69 Silver Palladium 47 46 Ag Pd 107.9 106.4 Gold Platinum 79 78 Au Pt 197.0 195.1 Unununium Ununnilium 111 110 Uuu Uun (272) (281) Gadolinium Europium 64 63 Gd Eu 157.3 152.0 Curium Americium 96 95 Cm Am (247) (243)
Periodic Table of the Elements Transition Elements VIIIB VIIIB (9) (8) Cobalt Iron 27 26 Co Fe 58.93 55.85 Rhodium Ruthenium 45 44 Rh Ru 102.9 101.1 Iridium Osmium 77 76 Ir Os 192.2 190.2 Meitnerium Hassium 109 108 Mt Hs (268) (277) Samarium Promethium 62 61 Sm Pm 150.4 (145) Plutonium Neptunium 94 93 Pu Np (244) (237) Values in parentheses are the mass numbers of the most stable or best-known isotopes. Names and symbols for elements 110–118 are under review.
VIB VIIB (7) (6) Manganese Chromium 25 24 Mn Cr 54.94 52.00 Technetium Molybdenum 43 42 Tc Mo (98) 95.94 Rhenium Tungsten 75 74 Re W 186.2 183.8 Bohrium Seaborgium 107 106 Bh Sg (264) (266) Neodymium Praseodymium 60 59 Nd Pr 144.2 140.9 Uranium Protactinium 92 91 U Pa 238.0 231.0
Semiconductors VB (5) Vanadium 23 V 50.94 Niobium 41 Nb 92.91 Tantalum 73 Ta 180.9 Dubnium 105 Db (262) Cerium 58 Ce 140.1 Thorium 90 Th 232.0 atomic number atomic weight
Metals Nonmetals IVB (4) Titanium 22 Ti 47.88 Zirconium 40 Zr 91.22 Hafnium 72 Hf 178.5 Rutherfordium 104 Rf (261)
† ‡ Inner Transition Elements † Lanthanides 6 ‡ Actinides 7 Hydrogen 1 H 1.008 The periodic table of the elements.
IIIB (3) Scandium 21 Sc 44.96 Yttrium 39 Y 88.91 Lanthanum 57 La 138.9 Actinium 89 Ac (227)
Alkaline Earth Metals IIA (2) Beryllium 4 Be 9.012 Magnesium 12 Mg 24.31 Calcium 20 Ca 40.08 Strontium 38 Sr 87.62 Barium 56 Ba 137.3 Radium 88 Ra (226) element name symbol of element
Alkali Metals IA (1) Hydrogen 1 H 1.008 Lithium 3 Li 6.941 Sodium 11 Na 22.99 Potassium 19 K 39.10 Rubidium 37 Rb 85.47 Cesium 55 Cs 132.9 Francium 87 Fr (223) Key FIGURE 8.17
Period 1 2 3 4 5 6 7
8-15 CHAPTER 8 Atoms and Periodic Properties 217