Page 303 - 9780077418427.pdf
P. 303
/Users/user-f465/Desktop
tiL12214_ch11_275-298.indd Page 280 9/3/10 6:12 PM user-f465
tiL12214_ch11_275-298.indd Page 280 9/3/10 6:12 PM user-f465 /Users/user-f465/Desktop
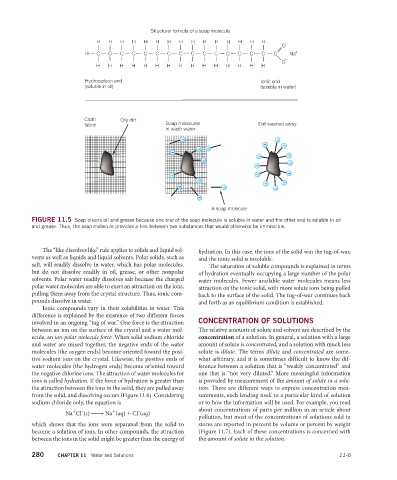
Structural formula of a soap molecule
H H H H H H H H H H H H H H H
O
H C C C C C C C C C C C C C C C C Na +
–
O
H H H H H H H H H H H H H H H
Hydrocarbon end Ionic end
(soluble in oil) (soluble in water)
Cloth Oily dirt
fabric Soap molecules Dirt washed away
in wash water
– –
– –
– –
– – –
–
– –
– – –
– –
– – – –
–
A soap molecule
FIGURE 11.5 Soap cleans oil and grease because one end of the soap molecule is soluble in water and the other end is soluble in oil
and grease. Thus, the soap molecule provides a link between two substances that would otherwise be immiscible.
The “like dissolves like” rule applies to solids and liquid sol- hydration. In this case, the ions of the solid win the tug-of-war,
vents as well as liquids and liquid solvents. Polar solids, such as and the ionic solid is insoluble.
salt, will readily dissolve in water, which has polar molecules, The saturation of soluble compounds is explained in terms
but do not dissolve readily in oil, grease, or other nonpolar of hydration eventually occupying a large number of the polar
solvents. Polar water readily dissolves salt because the charged water molecules. Fewer available water molecules means less
polar water molecules are able to exert an attraction on the ions, attraction on the ionic solid, with more solute ions being pulled
pulling them away from the crystal structure. Thus, ionic com- back to the surface of the solid. The tug-of-war continues back
pounds dissolve in water. and forth as an equilibrium condition is established.
Ionic compounds vary in their solubilities in water. This
difference is explained by the existence of two different forces
involved in an ongoing “tug of war.” One force is the attraction CONCENTRATION OF SOLUTIONS
between an ion on the surface of the crystal and a water mol- The relative amounts of solute and solvent are described by the
ecule, an ion-polar molecule force. When solid sodium chloride concentration of a solution. In general, a solution with a large
and water are mixed together, the negative ends of the water amount of solute is concentrated, and a solution with much less
molecules (the oxygen ends) become oriented toward the posi- solute is dilute. The terms dilute and concentrated are some-
tive sodium ions on the crystal. Likewise, the positive ends of what arbitrary, and it is sometimes difficult to know the dif-
water molecules (the hydrogen ends) become oriented toward ference between a solution that is “weakly concentrated” and
the negative chlorine ions. The attraction of water molecules for one that is “not very diluted.” More meaningful information
ions is called hydration. If the force of hydration is greater than is provided by measurement of the amount of solute in a solu-
the attraction between the ions in the solid, they are pulled away tion. There are different ways to express concentration mea-
from the solid, and dissolving occurs (Figure 11.6). Considering surements, each lending itself to a particular kind of solution
sodium chloride only, the equation is or to how the information will be used. For example, you read
+ – + – about concentrations of parts per million in an article about
Na Cl (s) → Na (aq) + Cl (aq )
pollution, but most of the concentrations of solutions sold in
which shows that the ions were separated from the solid to stores are reported in percent by volume or percent by weight
become a solution of ions. In other compounds, the attraction (Figure 11.7). Each of these concentrations is concerned with
between the ions in the solid might be greater than the energy of the amount of solute in the solution.
280 CHAPTER 11 Water and Solutions 11-6