Page 307 - 9780077418427.pdf
P. 307
/Users/user-f465/Desktop
tiL12214_ch11_275-298.indd Page 284 9/3/10 6:12 PM user-f465
tiL12214_ch11_275-298.indd Page 284 9/3/10 6:12 PM user-f465 /Users/user-f465/Desktop
150
140
130
120
9
110 NaNO 3 A volts
Solubility (g solute/100 g water) 90 KNO 3 KCl
100
80
70
60
50
40
NaCl
9
30 volts
20
B
10
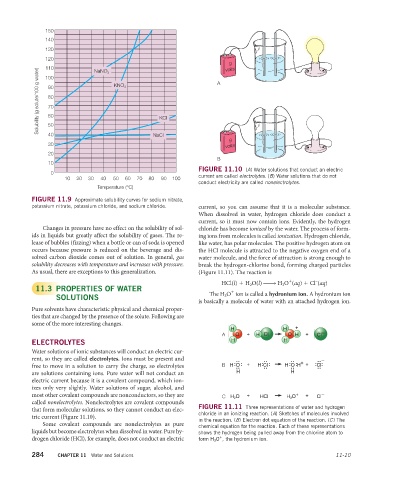
0 FIGURE 11.10 (A) Water solutions that conduct an electric
10 20 30 40 50 60 70 80 90 100 current are called electrolytes. (B) Water solutions that do not
conduct electricity are called nonelectrolytes.
Temperature (°C)
FIGURE 11.9 Approximate solubility curves for sodium nitrate,
potassium nitrate, potassium chloride, and sodium chloride. current, so you can assume that it is a molecular substance.
When dissolved in water, hydrogen chloride does conduct a
current, so it must now contain ions. Evidently, the hydrogen
Changes in pressure have no effect on the solubility of sol- chloride has become ionized by the water. The process of form-
ids in liquids but greatly affect the solubility of gases. The re- ing ions from molecules is called ionization. Hydrogen chloride,
lease of bubbles (fizzing) when a bottle or can of soda is opened like water, has polar molecules. The positive hydrogen atom on
occurs because pressure is reduced on the bever age and dis- the HCl molecule is attracted to the negative oxygen end of a
solved carbon dioxide comes out of solution. In general, gas water molecule, and the force of attraction is strong enough to
solubility decreases with temperature and increases with pressure. break the hydrogen-chlorine bond, forming charged particles
As usual, there are exceptions to this generalization. (Figure 11.11). The reaction is
+ –
HCl(l) + H 2 O(l) → H 3 O (aq) + Cl (aq)
11.3 PROPERTIES OF WATER +
The H 3 O ion is called a hydronium ion. A hydronium ion
SOLUTIONS
is basically a molecule of water with an attached hydrogen ion.
Pure solvents have characteristic physical and chemical proper-
ties that are changed by the presence of the solute. Following are
some of the more interesting changes.
H H +
A O + H Cl O H + Cl –
ELECTROLYTES H H
Water solutions of ionic substances will conduct an electric cur-
rent, so they are called electrolytes. Ions must be present and .... .... .. .. + .. ..–
..
O H
Cl
free to move in a solution to carry the charge, so electrolytes B H O .. + H .. .. H .. .. + Cl .. ..
are solutions containing ions. Pure water will not conduct an H H
electric current because it is a covalent compound, which ion-
izes only very slightly. Water solutions of sugar, alcohol, and
most other covalent compounds are nonconductors, so they are C H 2 O + HCl H 3 O + + Cl –
called nonelectrolytes. Nonelectrolytes are covalent compounds
that form molecular solutions, so they cannot conduct an elec- FIGURE 11.11 Three representations of water and hydrogen
chloride in an ionizing reaction. (A) Sketches of molecules involved
tric current (Figure 11.10).
in the reaction. (B) Electron dot equation of the reaction. (C) The
Some covalent compounds are nonelectrolytes as pure chemical equation for the reaction. Each of these representations
liquids but become electrolytes when dissolved in water. Pure hy- shows the hydrogen being pulled away from the chlorine atom to
+
drogen chloride (HCl), for example, does not conduct an electric form H 3 O , the hydronium ion.
284 CHAPTER 11 Water and Solutions 11-10