Page 48 - ACE YR IGCSE A TOP APPROACH TO CHEM
P. 48
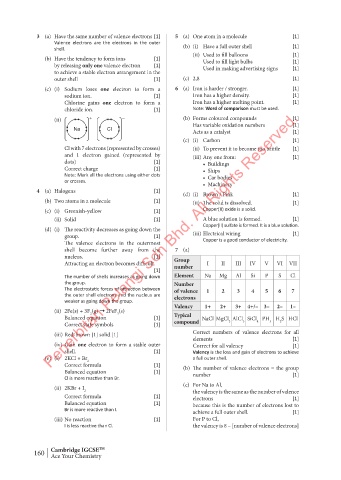
3 (a) Have the same number of valence electrons [1] 5 (a) One atom in a molecule [1]
Valence electrons are the electrons in the outer
shell. (b) (i) Have a full outer shell [1]
(ii) Used to fill balloons [1]
(b) Have the tendency to form ions [1] Used to fill light bulbs [1]
by releasing only one valence electron [1] Used in making advertising signs [1]
to achieve a stable electron arrangement in the
outer shell [1] (c) 2,8 [1]
(c) (i) Sodium loses one electron to form a 6 (a) Iron is harder / stronger. [1]
sodium ion. [1] Iron has a higher density. [1]
Chlorine gains one electron to form a Iron has a higher melting point. [1]
chloride ion. [1] Note: Word of comparison must be used.
(ii) + – (b) Forms coloured compounds [1]
Has variable oxidation numbers [1]
Na Cl
Acts as a catalyst [1]
(c) (i) Carbon [1]
Cl with 7 electrons (represented by crosses) (ii) To prevent it to become too brittle [1]
and 1 electron gained (represented by (iii) Any one from: [1]
dots) [1] • Buildings
Correct charge [1] • Ships
Note: Mark all the electrons using either dots • Car bodies
or crosses.
• Machinery
4 (a) Halogens [1]
(d) (i) Brown / Pink [1]
(b) Two atoms in a molecule [1] (ii) The solid is dissolved. [1]
(c) (i) Greenish-yellow [1] Copper(II) oxide is a solid.
(ii) Solid [1] A blue solution is formed. [1]
Copper(II) sulfate is formed. It is a blue solution.
(d) (i) The reactivity decreases as going down the
group. [1] (iii) Electrical wiring [1]
Copper is a good conductor of electricity.
Penerbitan Pelangi Sdn Bhd. All Rights Reserved.
The valence electrons in the outermost
shell become further away from the 7 (a)
nucleus. [1]
Attracting an electron becomes difficult. Group I II III IV V VI VII
number
[1]
The number of shells increases as going down Element Na Mg Al Si P S Cl
the group. Number
The electrostatic forces of attraction between of valence 1 2 3 4 5 6 7
the outer shell electrons and the nucleus are electrons
weaker as going down the group.
Valency 1+ 2+ 3+ 4+/– 3– 2– 1–
(ii) 2Fe(s) + 3F (g) ➞ 2FeF (s)
3
2
Balanced equation [1] Typical NaCl MgCl AlCl SiCl PH H S HCl
Correct state symbols [1] compound 2 3 4 3 2
(iii) Red-brown [1] solid [1] Correct numbers of valence electrons for all
elements [1]
(iv) Gain one electron to form a stable outer Correct for all valency [1]
shell. [1] Valency is the loss and gain of electrons to achieve
(e) (i) 2KCl + Br 2 a full outer shell.
Correct formula [1] (b) The number of valence electrons = the group
Balanced equation [1] number [1]
Cl is more reactive than Br.
(c) For Na to Al,
(ii) 2KBr + I 2 the valency is the same as the number of valence
Correct formula [1] electrons [1]
Balanced equation [1] because this is the number of electrons lost to
Br is more reactive than I. achieve a full outer shell. [1]
(iii) No reaction [1] For P to Cl,
I is less reactive than Cl. the valency is 8 – [number of valence electrons]
Cambridge IGCSE TM
160 Ace Your Chemistry
Answers.indd 160 3/4/22 3:54 PM