Page 14 - Focus SPM KSSM F4 2020 - Chemistry
P. 14
Chemistry Form 4 Chapter 3 The Mole Concept, Chemical Formula and Equation
3.3 Chemical Formula
What is chemical formula?
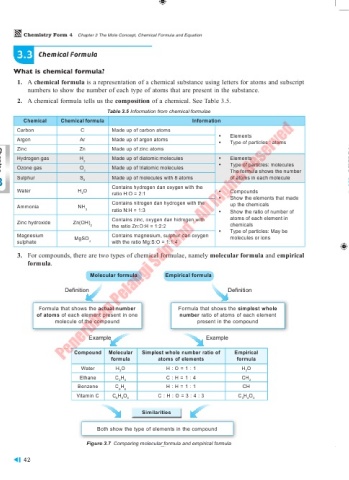
1. A chemical formula is a representation of a chemical substance using letters for atoms and subscript
numbers to show the number of each type of atoms that are present in the substance.
2. A chemical formula tells us the composition of a chemical. See Table 3.5.
Table 3.5 Information from chemical formulae
Chemical Chemical formula Information
Carbon C Made up of carbon atoms
Argon Ar Made up of argon atoms • Elements
• Type of particles : atoms
Zinc Zn Made up of zinc atoms
Hydrogen gas H Made up of diatomic molecules • Elements
2
Ozone gas O Made up of triatomic molecules • Type of particles: molecules
3 The formula shows the number
Chapter
3 Sulphur S 8 Made up of molecules with 8 atoms of atoms in each molecule
Water H O Contains hydrogen dan oxygen with the Compounds
2 ratio H:O = 2:1 •
• Show the elements that made
Contains nitrogen dan hydrogen with the up the chemicals
Ammonia NH
3 ratio N:H = 1:3 • Show the ratio of number of
Contains zinc, oxygen dan hidrogen with atoms of each element in
Zinc hydroxide Zn(OH) 2 the ratio Zn:O:H = 1:2:2 chemicals
• Type of particles: May be
Magnesium MgSO Contains magnesium, sulphur dan oxygen molecules or ions
sulphate 4 with the ratio Mg:S:O = 1:1:4
3. For compounds, there are two types of chemical formulae, namely molecular formula and empirical
formula.
Molecular formula Empirical formula
Definition Definition
Formula that shows the actual number Formula that shows the simplest whole
of atoms of each element present in one number ratio of atoms of each element
molecule of the compound present in the compound
Example Example
Compound Molecular Simplest whole number ratio of Empirical
formula atoms of elements formula
Water H O H : O = 1 : 1 H O
2
2
Ethane C H C : H = 1 : 4 CH
2 4 2
Benzene C H H : H = 1 : 1 CH
6 6
Vitamin C C H O C : H : O = 3 : 4 : 3 C H O
6 8 6 3 4 3
Similarities
Both show the type of elements in the compound
Figure 3.7 Comparing molecular formula and empirical formula
42
03 SPM CHEMISTRY F4.indd 42 27/02/2020 11:23 AM