Page 19 - Focus SPM KSSM F4 2020 - Chemistry
P. 19
Chemistry Form 4 Chapter 3 The Mole Concept, Chemical Formula and Equation
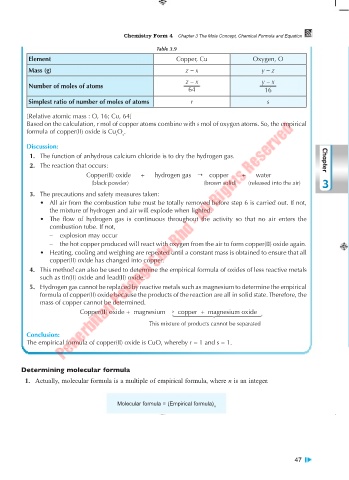
Table 3.9
Element Copper, Cu Oxygen, O
Mass (g) z – x y – z
z – x y – x
Number of moles of atoms
64 16
Simplest ratio of number of moles of atoms r s
[Relative atomic mass : O, 16; Cu, 64]
Based on the calculation, r mol of copper atoms combine with s mol of oxygen atoms. So, the empirical
formula of copper(II) oxide is Cu O .
r s
Discussion:
1. The function of anhydrous calcium chloride is to dry the hydrogen gas.
2. The reaction that occurs: Chapter
Copper(II) oxide + hydrogen gas → copper + water
(black powder) (brown solid) (released into the air) 3
3. The precautions and safety measures taken:
• All air from the combustion tube must be totally removed before step 6 is carried out. If not,
the mixture of hydrogen and air will explode when lighted.
• The flow of hydrogen gas is continuous throughout the activity so that no air enters the
combustion tube. If not,
– explosion may occur
– the hot copper produced will react with oxygen from the air to form copper(II) oxide again.
• Heating, cooling and weighing are repeated until a constant mass is obtained to ensure that all
copper(II) oxide has changed into copper.
4. This method can also be used to determine the empirical formula of oxides of less reactive metals
such as tin(II) oxide and lead(II) oxide.
5. Hydrogen gas cannot be replaced by reactive metals such as magnesium to determine the empirical
formula of copper(II) oxide because the products of the reaction are all in solid state. Therefore, the
mass of copper cannot be determined.
Copper(II) oxide + magnesium → copper + magnesium oxide
This mixture of products cannot be separated
Conclusion:
The empirical formula of copper(II) oxide is CuO, whereby r = 1 and s = 1.
Determining molecular formula
1. Actually, molecular formula is a multiple of empirical formula, where n is an integer.
Molecular formula = (Empirical formula)
n
47
03 SPM CHEMISTRY F4.indd 47 27/02/2020 11:23 AM