Page 16 - Focus SPM KSSM F4 2020 - Chemistry
P. 16
Chemistry Form 4 Chapter 3 The Mole Concept, Chemical Formula and Equation
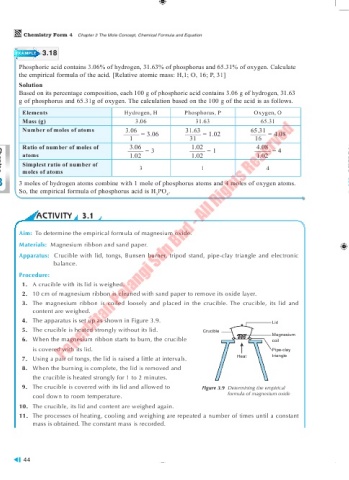
EXAMPLE 3.18
Phosphoric acid contains 3.06% of hydrogen, 31.63% of phosphorus and 65.31% of oxygen. Calculate
the empirical formula of the acid. [Relative atomic mass: H,1; O, 16; P, 31]
Solution
Based on its percentage composition, each 100 g of phosphoric acid contains 3.06 g of hydrogen, 31.63
g of phosphorus and 65.31g of oxygen. The calculation based on the 100 g of the acid is as follows.
Elements Hydrogen, H Phosphorus, P Oxygen, O
Mass (g) 3.06 31.63 65.31
Number of moles of atoms 3.06 = 3.06 31.63 = 1.02 65.31 = 4.08
1 31 16
Ratio of number of moles of 3.06 = 3 1.02 = 1 4.08 = 4
atoms 1.02 1.02 1.02
Simplest ratio of number of 3 1 4
moles of atoms
Chapter
3 3 moles of hydrogen atoms combine with 1 mole of phosphorus atoms and 4 moles of oxygen atoms.
So, the empirical formula of phosphorus acid is H PO .
3 4
ACTIVITY 3.1
Aim: To determine the empirical formula of magnesium oxide.
Materials: Magnesium ribbon and sand paper.
Apparatus: Crucible with lid, tongs, Bunsen burner, tripod stand, pipe-clay triangle and electronic
balance.
Procedure:
1. A crucible with its lid is weighed.
2. 10 cm of magnesium ribbon is cleaned with sand paper to remove its oxide layer.
3. The magnesium ribbon is coiled loosely and placed in the crucible. The crucible, its lid and
content are weighed.
4. The apparatus is set up as shown in Figure 3.9. Lid
5. The crucible is heated strongly without its lid. Crucible
Magnesium
6. When the magnesium ribbon starts to burn, the crucible coil
is covered with its lid. Pipe-clay
7. Using a pair of tongs, the lid is raised a little at intervals. Heat triangle
8. When the burning is complete, the lid is removed and
the crucible is heated strongly for 1 to 2 minutes.
9. The crucible is covered with its lid and allowed to Figure 3.9 Determining the empirical
cool down to room temperature. formula of magnesium oxide
10. The crucible, its lid and content are weighed again.
11. The processes of heating, cooling and weighing are repeated a number of times until a constant
mass is obtained. The constant mass is recorded.
44
03 SPM CHEMISTRY F4.indd 44 27/02/2020 11:23 AM