Page 15 - Focus SPM KSSM F4 2020 - Chemistry
P. 15
Chemistry Form 4 Chapter 3 The Mole Concept, Chemical Formula and Equation
Determining empirical formula
1. The empirical formula of a compound is determined by investigating the simplest ratio of the number
of moles of the elements in the compound in a chemical laboratory.
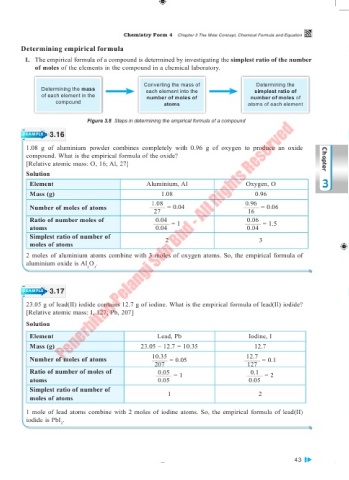
Converting the mass of Determining the
Determining the mass each element into the simplest ratio of
of each element in the number of moles of number of moles of
compound atoms atoms of each element
Figure 3.8 Steps in determining the empirical formula of a compound
EXAMPLE 3.16
1.08 g of aluminium powder combines completely with 0.96 g of oxygen to produce an oxide
compound. What is the empirical formula of the oxide?
[Relative atomic mass: O, 16; Al, 27] Chapter
Solution
Element Aluminium, Al Oxygen, O 3
Mass (g) 1.08 0.96
1.08 0.96
Number of moles of atoms = 0.04 = 0.06
27 16
Ratio of number moles of 0.04 = 1 0.06 = 1.5
atoms 0.04 0.04
Simplest ratio of number of 2 3
moles of atoms
2 moles of aluminium atoms combine with 3 moles of oxygen atoms. So, the empirical formula of
aluminium oxide is Al O .
2 3
EXAMPLE 3.17
23.05 g of lead(II) iodide contains 12.7 g of iodine. What is the empirical formula of lead(II) iodide?
[Relative atomic mass: I, 127; Pb, 207]
Solution
Element Lead, Pb Iodine, I
Mass (g) 23.05 – 12.7 = 10.35 12.7
Number of moles of atoms 10.35 = 0.05 12.7 = 0.1
207 127
Ratio of number of moles of 0.05 = 1 0.1 = 2
atoms 0.05 0.05
Simplest ratio of number of 1 2
moles of atoms
1 mole of lead atoms combine with 2 moles of iodine atoms. So, the empirical formula of lead(II)
iodide is PbI .
2
43
03 SPM CHEMISTRY F4.indd 43 27/02/2020 11:23 AM