Page 21 - Focus SPM KSSM F4 2020 - Chemistry
P. 21
Chemistry Form 4 Chapter 3 The Mole Concept, Chemical Formula and Equation
SPM Highlights
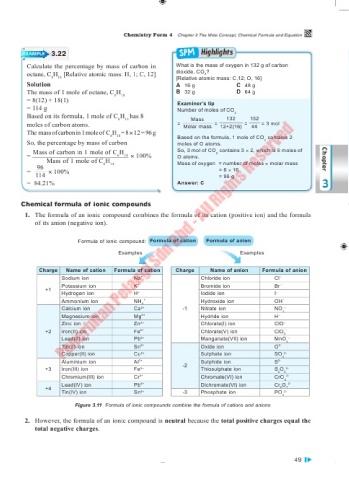
EXAMPLE 3.22
Calculate the percentage by mass of carbon in What is the mass of oxygen in 132 g of carbon
octane, C H [Relative atomic mass: H, 1; C, 12] dioxide, CO ?
2
18
[Relative atomic mass: C,12; O, 16]
8
Solution A 16 g C 48 g
The mass of 1 mole of octane, C H 18 B 32 g D 64 g
8
= 8(12) + 18(1) Examiner’s tip
= 114 g Number of moles of CO
Based on its formula, 1 mole of C H has 8 Mass 132 2 132
18
8
moles of carbon atoms. = Molar mass = 12+2(16) = 44 = 3 mol
The mass of carbon in 1 mole of C H = 8 × 12 = 96 g Based on the formula, 1 mole of CO contains 2
8
18
So, the percentage by mass of carbon moles of O atoms. 2
= Mass of carbon in 1 mole of C H 14 × 100% So, 3 mol of CO contains 3 × 2, which is 6 moles of
2
6
O atoms.
Mass of 1 mole of C H Mass of oxygen = number of moles × molar mass Chapter
96 6 14
= 114 × 100% = 6 × 16
= 96 g
= 84.21% Answer: C 3
Chemical formula of ionic compounds
1. The formula of an ionic compound combines the formula of its cation (positive ion) and the formula
of its anion (negative ion).
Formula of ionic compound: Formula of cation Formula of anion
Examples Examples
Charge Name of cation Formula of cation Charge Name of anion Formula of anion
Sodium ion Na + Chloride ion Cl –
Potassium ion K + Bromide ion Br –
+1
Hydrogen ion H + Iodide ion I –
Ammonium ion NH 4 + Hydroxide ion OH –
Calcium ion Ca 2+ -1 Nitrate ion NO –
3
Magnesium ion Mg 2+ Hydride ion H –
Zinc ion Zn 2+ Chlorate(I) ion ClO –
+2 Iron(II) ion Fe 2+ Chlorate(V) ion ClO 3 –
Lead(II) ion Pb 2+ Manganate(VII) ion MnO 4 –
Tin(II) ion Sn 2+ Oxide ion O
2-
Copper(II) ion Cu 2+ Sulphate ion SO
2-
4
Aluminium ion Al 3+ Sulphide ion S
2-
+3 Iron(III) ion Fe 3+ -2 Thiosulphate ion S O
2-
2 3
Chromium(III) ion Cr 3+ Chromate(VI) ion CrO
2-
4
Lead(IV) ion Pb 4+ Dichromate(VI) ion Cr O
2-
+4 4+ 2 3- 7
Tin(IV) ion Sn -3 Phosphate ion PO
4
Figure 3.11 Formula of ionic compounds combine the formula of cations and anions
2. However, the formula of an ionic compound is neutral because the total positive charges equal the
total negative charges.
49
03 SPM CHEMISTRY F4.indd 49 27/02/2020 11:23 AM