Page 20 - Focus SPM KSSM F4 2020 - Chemistry
P. 20
Chemistry Form 4 Chapter 3 The Mole Concept, Chemical Formula and Equation
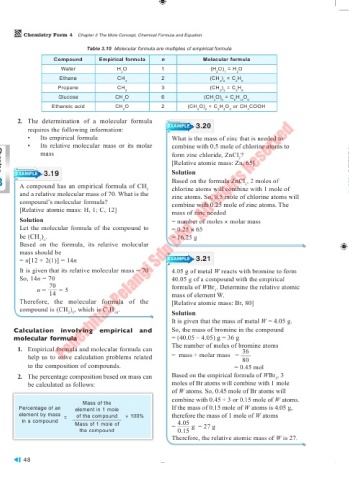
Table 3.10 Molecular formula are multiples of empirical formula
Compound Empirical formula n Molecular formula
Water H O 1 (H O) = H O
2 2 1 2
Ethane CH 2 (CH ) = C H
3 3 2 2 6
Propane CH 3 (CH ) = C H
2 2 3 3 6
Glucose CH O 6 (CH O) = C H O
2 2 6 6 12 6
Ethanoic acid CH O 2 (CH O) = C H O or CH COOH
2 2 2 2 4 2 3
2. The determination of a molecular formula
requires the following information: EXAMPLE 3.20
• Its empirical formula What is the mass of zinc that is needed to
• Its relative molecular mass or its molar combine with 0.5 mole of chlorine atoms to
mass form zinc chloride, ZnCl ?
2
[Relative atomic mass: Zn, 65]
EXAMPLE 3.19 Solution
Chapter
3 A compound has an empirical formula of CH Based on the formula ZnCl , 2 moles of
2
2
and a relative molecular mass of 70. What is the chlorine atoms will combine with 1 mole of
zinc atoms. So, 0.5 mole of chlorine atoms will
compound’s molecular formula? combine with 0.25 mole of zinc atoms. The
[Relative atomic mass: H, 1; C, 12] mass of zinc needed
Solution = number of moles × molar mass
Let the molecular formula of the compound to = 0.25 × 65
be (CH ) . = 16.25 g
2 n
Based on the formula, its relative molecular
mass should be
= n[12 + 2(1)] = 14n EXAMPLE 3.21
It is given that its relative molecular mass = 70 4.05 g of metal W reacts with bromine to form
So, 14n = 70 40.05 g of a compound with the empirical
70 formula of WBr . Determine the relative atomic
n = = 5 3
14 mass of element W.
Therefore, the molecular formula of the [Relative atomic mass: Br, 80]
compound is (CH ) , which is C H . Solution
5
10
2 5
It is given that the mass of metal W = 4.05 g.
Calculation involving empirical and So, the mass of bromine in the compound
molecular formula = (40.05 – 4.05) g = 36 g
1. Empirical formula and molecular formula can The number of moles of bromine atoms
36
help us to solve calculation problems related = mass ÷ molar mass = 80
to the composition of compounds. = 0.45 mol
2. The percentage composition based on mass can Based on the empirical formula of WBr , 3
3
be calculated as follows: moles of Br atoms will combine with 1 mole
of W atoms. So, 0.45 mole of Br atoms will
combine with 0.45 ÷ 3 or 0.15 mole of W atoms.
Mass of the
Percentage of an element in 1 mole If the mass of 0.15 mole of W atoms is 4.05 g,
element by mass = of the compound × 100% therefore the mass of 1 mole of W atoms
in a compound
Mass of 1 mole of = 4.05 g = 27 g
the compound 0.15
Therefore, the relative atomic mass of W is 27.
48
03 SPM CHEMISTRY F4.indd 48 27/02/2020 11:23 AM