Page 29 - APS(2022)_TG5 _Edisi Guru
P. 29
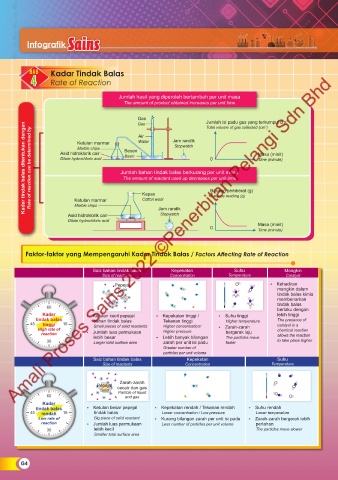
Infografik Sains
BAB Kadar Tindak Balas
4 4 Rate of Reaction
Jumlah hasil yang diperoleh bertambah per unit masa
The amount of product obtained increases per unit time
Gas Jam randik Jumlah isi padu gas yang terkumpul cm 3
Kadar tindak balas ditentukan dengan Rate of reaction can be determined by Asid hidroklorik cair Jumlah bahan tindak balas berkurang per unit masa Masa (minit)
Gas
Total volume of gas collected (cm )
3
Air
Water
Ketulan marmar
Stopwatch
Marble chips
Besen
Basin
55
5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5
55
5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5
55
55
10
10
0
0
0
10
1 1 1 1 10
10
10
10
10
10
N
I
5 5
M I N
M
50
50
25
25
0
10
0
0
10
0
10
10
0
10
10
10
10
10
10
10
10
10
10
0
0
0
0
0
0
1 1 1 1 10
0
0
0
25
50
M
M I N
25
10
10
10
50
10
10
10
10
10
10
5 5
N
10
I
Dilute hydrochloric acid
15
15
20
10
10
20
10
15
10
20
20
15
5
5
5
5
5
5
5
Time (minute)
5
5
5
5
5
5
5
5
5
15
5
5
5
5
5
5
5
5
5
5
5
15
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
45
5
5
5
5
45
5
5
15
5
5
5
15
5
45
45
20
40
20
40
40
20
20
40
25
25
30
30
30
30
25
25
35
35
35
35
The amount of reactant used up decreases per unit time
Bacaan pemberat (g)
Ketulan marmar
Cotton wool
Marble chips
Stopwatch
Asid hidroklorik cair Kapas Jam randik Balance reading (g)
Dilute hydrochloric acid
10.00 g 50 50 45 45 55 55 10.00 g 45 45 50 50 40 40 55 55 25 25 20 20 M M I NN I 15 15 5 5 10 10 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 0 0 10 10 10 0 0 0 0 0 0 10 10 10 10 1 1 1 1 1 1 1 10 10 10 10 10 10 10 15 15 20 20 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 0 Masa (minit)
5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5
10
0
10
25
10
N
M I N
I
5 5
M
0
0
0
0
10
10
10
0
10
10
10
1 1 1 1 1 1 1 10
0
10
10
10
25
0
10
10
10
20
20
5
5
5
5
5
5
5
15
5
5
5
15
5
5
15
5
5
5
5
5
5
5
5
5
5
5
15
5
5
5
5
40 40 20 20 35 35 30 30 25 25 Time (minute)
35 35 30 30 25 25
Faktor-faktor yang Mempengaruhi Kadar Tindak Balas / Factors Affecting Rate of Reaction
Saiz bahan tindak balas Kepekatan Suhu Mangkin
Size of reactants Concentration Temperature Catalyst
Pepejal • Kehadiran
Solid mangkin dalam
tindak balas kimia
membenarkan
tindak balas
60 berlaku dengan
Kadar • Ketulan kecil pepejal • Kepekatan tinggi / • Suhu tinggi lebih tinggi
tindak balas bahan tindak balas Tekanan tinggi Higher temperature The presence of
45 tinggi 15 Small pieces of solid reactants Higher concentration/ • Zarah-zarah catalyst in a
High rate of • Jumlah luas permukaan Higher pressure bergerak laju chemical reaction
reaction lebih besar • Lebih banyak bilangan allows the reaction
30 Larger total surface area zarah per unit isi padu The particles move to take place higher
faster
Greater number of
particles per unit volume
Saiz bahan tindak balas Kepekatan Suhu
Size of reactants Concentration Temperature
Zarah-zarah
Pepejal cecair dan gas
Solid Particle of liquid
60 and gas
Kadar
tindak balas • Ketulan besar pepejal • Kepekatan rendah / Tekanan rendah • Suhu rendah
45 rendah 15 tindak balas Lower concentration / Low pressure Lower temperature
Low rate of Big piece of solid reactant • Kurang bilangan zarah per unit isi padu • Zarah-zarah bergerak lebih
reaction • Jumlah luas permukaan Less number of particles per unit volume perlahan
30 lebih kecil The particles move slower
Smaller total surface area
G4 GPB
Infographic Amali Proses Sains TG5.indd 4 7/28/21 2:26 PM