Page 24 - Human Environment Interface (3)
P. 24
Novel Fluorescent Probes for the Oxidative Milieu
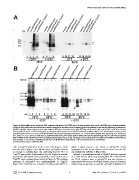
Figure 6. Formation of an aberrant SDS-resistant structure in SGFP2-prion fusion protein but not in cfSGFP2-prion fusion protein.
Lysates of cells expressing the prion fusion proteins indicated in the figure were analyzed by Western blotting as described in Materials and Methods.
(A) SDS-solubilized lysates were treated either without DTT (lanes1–4 and 9–12) or with DTT (lanes 5–8 and 13–16) at either 70uC or 95uC for 10 min.
Prion protein fused to ss-cfSGFP2 migrated on the gel at the expected size of 58 kDa under all conditions (lanes 2, 6, 10 and 14). In contrast, prion
protein fused to ss-SGFP2 or ss-SGFP2(C48S) showed intense fast migrating bands (lanes 3, 4, 7 and 8) when samples were treated at 70uC, but not at
95uC (lanes 11, 12, 15 and 16). * indicates an unknown form of the prion fusion protein which was not consistently observed. (B) Cells were incubated
with or without 100 nM MG132 for 4 hr as indicated in the figure and, as in (A), cell lysates were treated under four different conditions. When MG132
was included, a set of faster migrating bands was not diminished completely by a 95uC treatment (lane13, arrowhead).
doi:10.1371/journal.pone.0037551.g006
cells. ss-TagRFP synthesized in the treated cells showed a small which a signal sequence was added to cgfTagRFP clearly
but clear shift (‘‘aglyco’’, lane 10) whereas ss-cgfTagRFP showed highlighted the ER as did calnexin when export from the ER
no change in mobility (lane 12), confirming that the N71 of was inhibited by brefeldin A (Figure 8C).
TagRFP was actually N-glycosylated. Specific brightness analysis
showed that cgfTagRFP emits comparable amount of photons as mKate2, a near-infrared variant of TagRFP, has been reported
TagRFP upon excitation (lower panel of Figure 3). The emission as a useful tool for whole body imaging [41]. We confirmed that
spectrum of cgfTagRFP was undistinguishable to TagRFP the N71K mutation used in cgfTagRFP was also effective in
(Figure 8B). Transfection of a plasmid encoding ss-cgfTagRFP in retaining the fluorescence of mKate2, but the same set of cysteine
replacements as was done to generate cgfTagRFP abolished the
PLoS ONE | www.plosone.org 8 May 2012 | Volume 7 | Issue 5 | e37551