Page 519 - APPLIED PROCESS DESIGN FOR CHEMICAL AND PETROCHEMICAL PLANTS, Volume 1, 3rd Edition
P. 519
Applied Process Design 485
American Society for Testing Materials (ASTM) E-502, D- pressure rise may be a 40-fold increase [34]. There will be
56, D-92, D-93, D-1310, D-3278. no flame (except possibly a "cold flame," not examined
The flammable liquid itself does not burn; only the here) or explosion when the concentrations are outside
vapors emitted from the liquid burn. The vaporization of these flammability limits [32]. (See Sample listings of flam-
a liquid depends on its temperature and corresponding mability data Tables 7-20 and 7-21. Also see in Figures 7-45,
vapor pressure and increases as the temperature of the 7-46, and 7-47. The flammability limits are designated.
liquid increases. Thus, the warmer the liquid, the more
potentially hazardous it becomes. 1. Lower Flammability (Explosive) Limits ( LEL or IYL): The
lowest percentage concentration at which a flash or flame
Fire point: The lowest temperature at which a liquid in can develop and propagate from the source of ignition
an open container will give off enough vapors to contin- when in contact with a source of ignition in a combustible
ue to burn when once ignited [32]. This temperature is material.
generally somewhat above the open-cup flash point. 2. Upper Flammability (Explosive) Limits. ( UEL or UFL: The
highest percentage concentration at which a flash or
Ignition temperature: The minimum temperature to which flame can develop and propagate flame away from the
a material must be heated for it to ignite. Once an ignition source of ignition when in contact with a source of igni-
has occurred it will continue to burn until all the available tion in a combustible material. See Tables 7-20 and 7-21
fuel or oxidant has been consumed or until the flame is [34] for common flammable compounds.
extinguished by cooling or by some other means [34].
Figure 7-46 illustrates a typical relationship of limits
of flammability and ignitibility for a methane air mix-
Autoignition temperature: The lowest temperature of a
material required to initiate or cause self-sustained com- ture. Note that energy required to ignite a flammable
bustion in the absence of a spark or flame. This tempera- mixture (within its LEL and UEL) varies with the com-
ture can vary, depending on the substance, its size, and position, and that a 0.2 millijoule (mj) spark is inade-
the shape of the igniting surface or container and other quate to ignite even a stoichiometric mixture at atmos-
factors [32]. pheric pressure at 26°C, while 1-mj spark can ignite any
Spontaneous heating: Some flammable liquids combine
readily with oxygen in the air at ordinary temperatures
and give off heat. When the heat is generated faster than POSITIVE PHASE
it can be dissipated, the temperature rises and ignition or
the mixture may occur, as with liquids on waste or rubbish
or other materials [32].
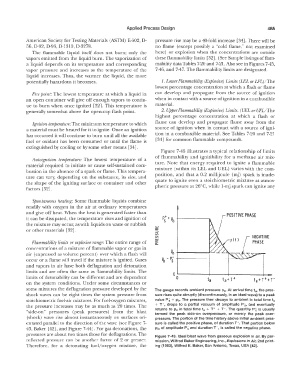
Flammability limits or explosive mnge: The entire range of
concentrations of a mixture of flammable vapor or gas in
air (expressed as volume percent) over which a flash will % +-����__.v_;
occur or a flame will travel if the mixture is ignited. Gases Po - ps-
and vapors in air have both deflagration and detonation
limits and are often the same as flammability limits. The
t--
limits of detonability can be different and are dependent 00'-- ���� ta � --jf-- ���� -+---
ta+T+
ta+T++r
on the system conditions. Under some circumstances or
some mixtures the deflagration pressure developed by the The gauge records ambient pressure p0• At arrival time ta, the pres-
shock waves can be eight times the system pressure from sure rises quite abruptly (discontinuously, in an ideal wave) to a peak
stoichiometric fuel-air mixtures. For fuel-oxygen mixtures, value P; + p0. The pressure then decays to ambient in total time ta
the pressure increases may be as much as 20 times. The + T+, drops to a partial vacuum of amplitude P;, and eventually
returns to Po in total time ta + T+ + T-. The quantity P� is usually
"side-on" pressures (peak pressures) from the blast termed the peak side-on overpressure, or merely the peak over-
(shock) wave rise almost instantaneously on surfaces ori- pressure. The portion of the time history above initial ambient pres-
entated parallel to the direction of the wave (see Figure 7- sure is called the positive phase, of duration T+. That portion below
43, Baker [ 42], and Figure 7-44). For gas detonations, the p0, of amplitude P� and duration T-, is called the negative phase.
pressures are about two times those for deflagrations. The Figure 7-43. Ideal blast wave from gaseous explosion in air. By per-
reflected pressure can be another factor of 2 or greater. mission, Wilfred Baker Engineering, Inc., Explosions in Air, 2nd print-
Therefore, for a detonating fuel/oxygen mixture, the ing (1983), Wilfred E. Baker, San Antonio, Texas, USA [42).