Page 518 - APPLIED PROCESS DESIGN FOR CHEMICAL AND PETROCHEMICAL PLANTS, Volume 1, 3rd Edition
P. 518
484 Applied Process Design for Chemical and Petrochemical Plants
pie, in a closed vessel, a hydrocarbon mixture detonation industry supported program is targeted to understanding
with air may generate 2.5 times (294 psi) a similar mixture the complexities of venting relief devices, particularly
in a deflagration. A deflagration may turn into a detona- from runaway reactors.
tion, especially if traveling down a long pipe. A shock wave
is generated by the expansion of gases created by the reac-
tion of one flammable gas/liquid with an oxidant, such as Terminology
air or pure oxygen, or an oxidizing material, or by pure
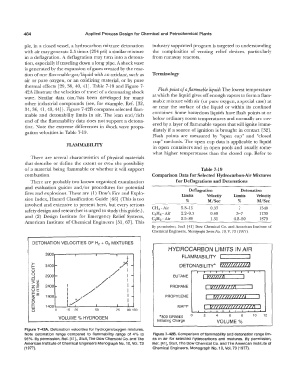
thermal effects [29, 38, 40, 41). Table 7-19 and Figure 7-
42A illustrate the velocities of travel of a detonating shock Flash point of a flammable liquid: The lowest temperature
wave. Similar data can/has been developed for many at which the liquid gives off enough vapors to form a flam-
other industrial compounds (see, for example, Ref. [32, mable mixture with air (or pure oxygen, a special case) at
34, 36, 41, 43, 44)). Figure 7-42B compares selected flam- or near the surface of the liquid or within its confined
mable and detonability limits in air. The lean end/rich container. Some hazardous liquids have flash points at or
end of the flammability data does not support a detona- below ordinary room temperatures and normally are cov-
tion. Note the extreme differences in shock wave propa- ered by a layer of flammable vapors that will ignite imme-
gation velocities in Table 7-19. diately if a source of ignition is brought in contact [32).
Flash points are measured by "open cup" and "closed
cup" methods. The open cup data is applicable to liquid
FLAMMABILITY
in open containers and in open pools and usually some-
what higher temperatures than the closed cup. Refer to
There are several characteristics of physical materials
that describe or define the extent or even the possibility
of a material being flammable or whether it will support Table 7-19
combustion. Comparison Data for Selected Hydrocarbon-Air Mixtures
There are probably two known organized examination for Deflagrarions and Detonations
and evaluation guides and/or procedures for potential Deflagration Detonation
fires and explosions. These are (1) Dow's Fire and Explo- Limits Velocity Limits Velocity
sion Index, Hazard Classification Guide [66) (This is too % M/Sec % M/Sec
involved and extensive to present here, but every serious
1540
?
safety design and researcher is urged to study this guide.), CH 4 -Air 5.3-15 0.37 3-7 1730
C3H8 -Air
2.2-9.5
0.40
and (2) Design Institute for Emergency Relief Systems, C2H2 -Air 2.5-80 1.31 4.2-50 1870
American Institute of Chemical Engineers [51, 67). This
By permission, Stull [ 41] Dow Chemical Co. and American Institute of
Chemical Engineers, Monograph Series No. 10, V. 73 (1977).
DETONATION VELOCITIES OF H 2 + 0 2 MIXTURES
HYDROCARBON LIMITS IN AIR
3900r-------T , ----------r,--------,,r--+.-- FLAMMABILITY
t'"""' _
>- +.)'¢1 DETONABILITY* r11oozom
I- 3400 -
0 /I
0 2900 ..... I -
...J
w. /+ I BUTANE I P1111nA
s s
Q)
z� 2400,- I - PROPANE v111110M
OE I / I
�-�
/
z 1900 1;_;,/ ' - PROPYLENE rllOWWZ!J
g 1400L---.;J,._ � �·....._ __.1 __. , __ __.1......., t1ommzaazzz1
I
Cl 0 15 25 50 75 90 100 MAPP
VOLUME % HYDROGEN *800 GRAMS 0 2 4 6 8 10 12
Initiating Charge VOLUME%
Figure 7-42A. Detonation velocities for hydrogen/oxygen mixtures.
Note detonation range compared to flammability range of 4% to Figure 7-428. Comparison of flammability and detonation range lim-
95%. By permission, Ref. (41)., Stull, The Dow Chemical Co. and The its in air for selected hydrocarbons and mixtures. By permission,
American Institute of Chemical Engineers Monograph No. 10, Vol. 73 Ref. (41), Stull, The Dow Chemical Co. and The American Institute of
(1977). Chemical Engineers, Monograph No. 10, Vol. 73 (1977).