Page 10 - 1202 Question Bank Chemistry Form 5 KSSM
P. 10
PAPER 3
0
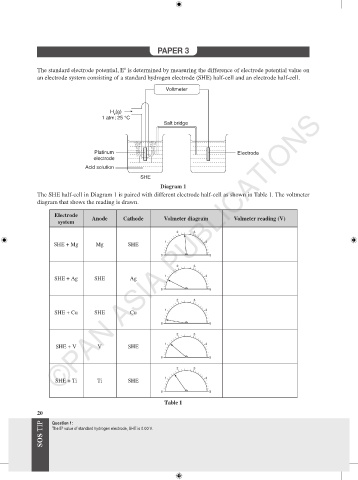
The standard electrode potential, E is determined by measuring the difference of electrode potential value on
an electrode system consisting of a standard hydrogen electrode (SHE) half-cell and an electrode half-cell.
Voltmeter
H (g)
©PAN ASIA PUBLICATIONS
2
1 atm; 25 °C
Salt bridge
Platinum Electrode
electrode
Acid solution
SHE
Diagram 1
The SHE half-cell in Diagram 1 is paired with different electrode half-cell as shown in Table 1. The voltmeter
diagram that shows the reading is drawn.
Electrode
Anode Cathode Volmeter diagram Volmeter reading (V)
system
2 3
1 4
SHE + Mg Mg SHE
0 5
2 3
1 4
SHE + Ag SHE Ag
0 5
2 3
SHE + Cu SHE Cu 1 4
0 5
2 3
1 4
SHE + V V SHE
0 5
2 3
SHE + Ti Ti SHE 1 4
0 5
Table 1
20 Question 1:
SOS TIP The E value of standard hydrogen electrode, SHE is 0.00 V.
0