Page 9 - 1202 Question Bank Chemistry Form 5 KSSM
P. 9
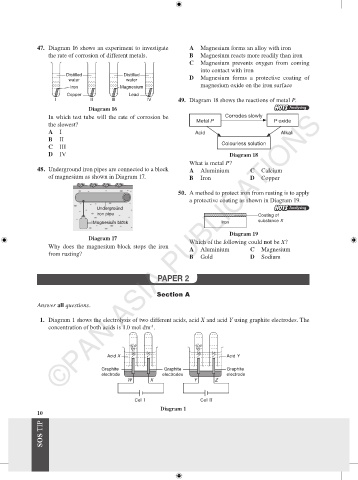
47. Diagram 16 shows an experiment to investigate A Magnesium forms an alloy with iron
the rate of corrosion of different metals. B Magnesium reacts more readily than iron
C Magnesium prevents oxygen from coming
into contact with iron
Distilled Distilled
water water D Magnesium forms a protective coating of
magnesium oxide on the iron surface
Iron Magnesium
Copper Lead
I II III IV 49. Diagram 18 shows the reactions of metal P.
Diagram 16 HOTS Analysing
©PAN ASIA PUBLICATIONS
In which test tube will the rate of corrosion be Corrodes slowly
Metal P P oxide
the slowest?
A I Acid Alkali
B II
Colourless solution
C III
D IV Diagram 18
What is metal P?
48. Underground iron pipes are connected to a block A Aluminium C Calcium
of magnesium as shown in Diagram 17. B Iron D Copper
50. A method to protect iron from rusting is to apply
a protective coating as shown in Diagram 19.
Underground HOTS Analysing
iron pipe Coating of
substance X
Magnesium block Iron
Diagram 19
Diagram 17
Which of the following could not be X?
Why does the magnesium block stops the iron A Aluminium C Magnesium
from rusting?
B Gold D Sodium
PAPER 2
Section A
Answer all questions.
1. Diagram 1 shows the electrolysis of two different acids, acid X and acid Y using graphite electrodes. The
concentration of both acids is 1.0 mol dm .
-3
Acid X Acid Y
Graphite Graphite Graphite
electrode electrodes electrode
W X Y Z
Cell I Cell II
Diagram 1
10
SOS TIP