Page 272 - Basic Principles of Textile Coloration
P. 272
MORDANT DYES FOR WOOL 261
NHCOCH3 SO2NHCH3 HH
O3S
N H O OH O H
N
O Cr SO3
O
O Cr O O
O N
N
N
N CI Acid Blue 158
CH3NHSO2
CH3CONH
CI Acid Black 60
CH3 SO3
NN
NN
O
O Cr O
O
O3S N N
NN
CH3
CI Acid Violet 90
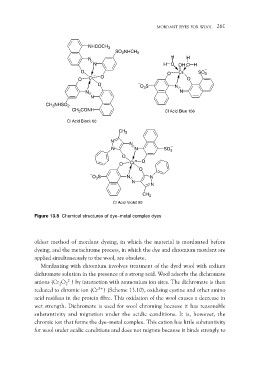
Figure 13.8 Chemical structures of dye–metal complex dyes
oldest method of mordant dyeing, in which the material is mordanted before
dyeing, and the metachrome process, in which the dye and chromium mordant are
applied simultaneously to the wool, are obsolete.
Mordanting with chromium involves treatment of the dyed wool with sodium
dichromate solution in the presence of a strong acid. Wool adsorbs the dichromate
anions (Cr2O72–) by interaction with ammonium ion sites. The dichromate is then
reduced to chromic ion (Cr3+) (Scheme 13.10), oxidising cystine and other amino
acid residues in the protein fibre. This oxidation of the wool causes a decrease in
wet strength. Dichromate is used for wool chroming because it has reasonable
substantivity and migration under the acidic conditions. It is, however, the
chromic ion that forms the dye–metal complex. This cation has little substantivity
for wool under acidic conditions and does not migrate because it binds strongly to