Page 271 - Basic Principles of Textile Coloration
P. 271
260 ACID, PRE-METALLISED AND MORDANT DYES
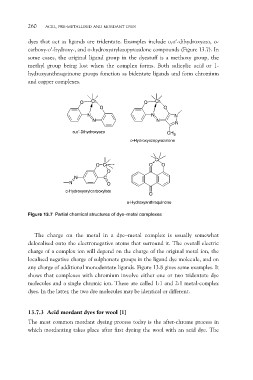
dyes that act as ligands are tridentate. Examples include o,o¢-dihydroxyazo, o-
carboxy-o¢-hydroxy-, and o-hydroxyarylazopyrazalone compounds (Figure 13.7). In
some cases, the original ligand group in the dyestuff is a methoxy group, the
methyl group being lost when the complex forms. Both salicylic acid or 1-
hydroxyanthraquinone groups function as bidentate ligands and form chromium
and copper complexes.
O Cr O Cr N
O O N
N N
N N
o,o′-Dihydroxyazo CH3
o-Hydroxyazopyrazalone
O Cr Cr
O OO
NC O
NO
o-Hydroxyanthraquinone
o-Hydroxyarylcarboxylate
Figure 13.7 Partial chemical structures of dye–metal complexes
The charge on the metal in a dye–metal complex is usually somewhat
delocalised onto the electronegative atoms that surround it. The overall electric
charge of a complex ion will depend on the charge of the original metal ion, the
localised negative charge of sulphonate groups in the ligand dye molecule, and on
any charge of additional monodentate ligands. Figure 13.8 gives some examples. It
shows that complexes with chromium involve either one or two tridentate dye
molecules and a single chromic ion. These are called 1:1 and 2:1 metal-complex
dyes. In the latter, the two dye molecules may be identical or different.
13.7.3 Acid mordant dyes for wool [1]
The most common mordant dyeing process today is the after-chrome process in
which mordanting takes place after first dyeing the wool with an acid dye. The