Page 270 - Basic Principles of Textile Coloration
P. 270
MORDANT DYES FOR WOOL 259
electron from a 3d to a vacant 4p orbital to give the electronic configuration
[Ar]4s03d84p1. This permits formation of four vacant dsp2 hybrid orbitals from the
vacant 3d, 4s and 4p orbitals, which results in a square-planar arrangement of four
ligand donors.
There are a number of aspects of coordination chemistry that Pauling’s theory
fails to explain and the newer ligand field theory has been more successful. For our
purposes, it suffices to recall that chromium has a coordination number of 6 and
that the ligands in a chromium complex give an octahedral arrangement around
the central chromium atom. Copper usually has a coordination number of 4 and
the four ligands form a square around the central copper atom.
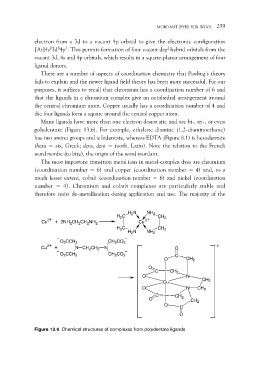
Many ligands have more than one electron donor site and are bi-, tri-, or even
polydentate (Figure 13.6). For example, ethylene diamine (1,2-diaminoethane)
has two amino groups and is bidentate, whereas EDTA (Figure 8.1) is hexadentate
(hexa = six, Greek; dens, dent = tooth, Latin). Note the relation to the French
word mordre (to bite), the origin of the word mordant.
The most important transition metal ions in metal-complex dyes are chromium
(coordination number = 6) and copper (coordination number = 4) and, to a
much lesser extent, cobalt (coordination number = 6) and nickel (coordination
number = 4). Chromium and cobalt complexes are particularly stable and
therefore resist de-metallisation during application and use. The majority of the
H2C H2N NH2 CH2
Cu 2
Cu2 + 2NH2CH2CH2NH2
H2C H2N NH2 CH2
Cu2 + O2CCH2 CH2CO2 2–
N CH2CH2 N O
O2CCH2 CH2CO2 O C CH2
O CH2 N CH2
C Cu
O N CH2
O CH2
C CH2
O OC
O
Figure 13.6 Chemical structures of complexes from polydentate ligands