Page 656 - Hall et al (2015) Principles of Critical Care-McGraw-Hill
P. 656
CHAPTER 53: Extracorporeal Lung Support 475
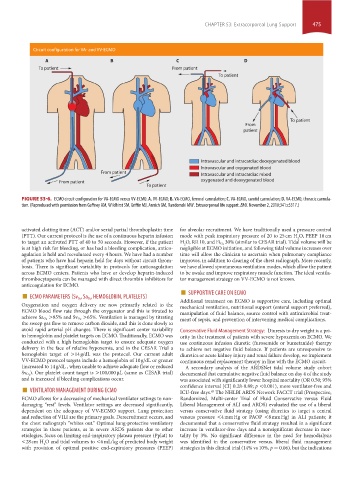
Circuit configuration for VA- and VV-ECMO
A B C D
To patient From patient
To patient
To patient
From
patient
Intravascular and intracardiac deoxygenated blood
Intravascular and oxygenated blood
From patient
Intravascular and intracardiac mixed
From patient oxygenated and deoxygenated blood
To patient
FIGURE 53-6. ECMO circuit configuration for VA-ECMO versus VV-ECMO. A. VV-ECMO; B. VA-ECMO, femoral cannulation; C. VA-ECMO, carotid cannulation; D. VA-ECMO, thoracic cannula-
tion. (Reproduced with permission from Gaffney AM, Wildhirst SM, Griffin MJ, Annich GM, Randomski MW. Extracorporeal life support. BMJ. November 2, 2010;341:c5317.)
activated clotting time (ACT) and/or serial partial thromboplastin time for alveolar recruitment. We have traditionally used a pressure control
(PTT). Our current protocol is the use of a continuous heparin infusion mode with peak inspiratory pressure of 20 to 25 cm H O, PEEP 10 cm
2
to target an activated PTT of 40 to 50 seconds. However, if the patient H O, RR 10, and Fi O 2 30% (similar to CESAR trial). Tidal volume will be
2
is at high risk for bleeding, or has had a bleeding complication, antico- negligible at ECMO initiation, and following tidal volume increases over
agulation is held and reevaluated every 4 hours. We have had a number time will allow the clinician to ascertain when pulmonary compliance
of patients who have had heparin held for days without circuit throm- improves, in addition to clearing of the chest radiograph. More recently,
bosis. There is significant variability in protocols for anticoagulation we have allowed spontaneous ventilation modes, which allow the patient
across ECMO centers. Patients who have or develop heparin-induced to be awake and improve respiratory muscle function. The ideal ventila-
thrombocytopenia can be managed with direct thrombin inhibitors for tor management strategy on VV-ECMO is not known.
anticoagulation for ECMO.
■ , HEMOGLOBIN, PLATELETS) ■ SUPPORTIVE CARE ON ECMO
, Sa O2
ECMO PARAMETERS (Sv O2
Oxygenation and oxygen delivery are now primarily related to the Additional treatment on ECMO is supportive care, including optimal
mechanical ventilation, nutritional support (enteral support preferred),
ECMO blood flow rate through the oxygenator and this is titrated to manipulation of fluid balance, source control with antimicrobial treat-
>65%. Ventilation is managed by titrating
achieve Sa O 2 >85% and Sv O 2 ment of sepsis, and prevention of intervening medical complications.
the sweep gas flow to remove carbon dioxide, and this is done slowly to
avoid rapid arterial pH changes. There is significant center variability Conservative Fluid Management Strategy: Diuresis to dry weight is a pri-
in hemoglobin and platelet targets on ECMO. Traditionally, ECMO was ority in the treatment of patients with severe hypoxemia on ECMO. We
conducted with a high hemoglobin target to ensure adequate oxygen use continuous infusion diuretic (furosemide or bumetanide) therapy
delivery in the face of relative hypoxemia, and in the CESAR Trial a to achieve net negative fluid balance. If patients are unresponsive to
hemoglobin target of >14 g/dL was the protocol. Our current adult diuretics or acute kidney injury and renal failure develop, we implement
VV-ECMO protocol targets include a hemoglobin of 10 g/dL or greater continuous renal replacement therapy in line with the ECMO circuit.
(increased to 14 g/dL , when unable to achieve adequate flow or reduced A secondary analysis of the ARDSNet tidal volume study cohort
). Our platelet count target is >100,000 μL (same as CESAR trial)
Sv O 2 documented that cumulative negative fluid balance on day 4 of the study
and is increased if bleeding complications occur. was associated with significantly lower hospital mortality (OR 0.50; 95%
■ VENTILATOR MANAGEMENT DURING ECMO confidence interval [CI] 0.28-0.89; p <0.001), more ventilator-free and
ICU-free days. The NHLBI ARDS Network FACCT trial (Prospective,
43
ECMO allows for a decreasing of mechanical ventilator settings to non- Randomized, Multi-center Trial of Fluid Conservative versus Fluid
damaging “rest” levels. Ventilator settings are decreased significantly, Liberal Management of ALI and ARDS) evaluated the use of a liberal
dependent on the adequacy of VV-ECMO support. Lung protection versus conservative fluid strategy (using diuretics to target a central
and reduction of VILI are the primary goals. Derecruitment occurs, and venous pressure <4 mm Hg or PAOP <8 mm Hg) in ALI patients; it
the chest radiograph “whites out.” Optimal lung-protective ventilatory documented that a conservative fluid strategy resulted in a significant
strategies in these patients, as in severe ARDS patients due to other increase in ventilator-free days and a nonsignificant decrease in mor-
etiologies, focus on limiting end-inspiratory plateau pressure (Pplat) to tality by 3%. No significant difference in the need for hemodialysis
<28 cm H O and tidal volumes to <6 mL/kg of predicted body weight was identified in the conservative versus. liberal fluid management
2
with provision of optimal positive end-expiratory pressures (PEEP) strategies in this clinical trial (14% vs 10%, p = 0.06), but the indications
section04.indd 475 1/23/2015 2:19:57 PM