Page 23 - The Netter Collection of Medical Illustrations - Integumentary System_ Volume 4 ( PDFDrive )
P. 23
Plate 1-8 Anatomy, Physiology, and Embryology
HEALING OF INCISED, SUTURED SKIN WOUND
WOUND HEALING
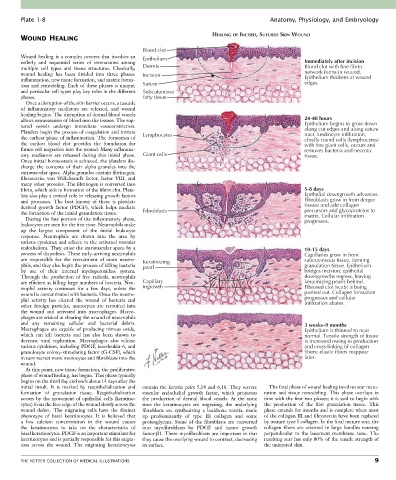
Blood clot
Wound healing is a complex process that involves an Epithelium
orderly and sequential series of interactions among Immediately after incision
multiple cell types and tissue structures. Classically, Dermis Blood clot with fine fibrin
wound healing has been divided into three phases: Incision network forms in wound.
inflammation, new tissue formation, and matrix forma- Epithelium thickens at wound
edges.
tion and remodeling. Each of these phases is unique, Suture
and particular cell types play key roles in the different Subcutaneous
phases. fatty tissue
Once a disruption of the skin barrier occurs, a cascade
of inflammatory mediators are released, and wound
healing begins. The disruption of dermal blood vessels
allows extravasation of blood into the tissues. The rup- 24-48 hours
tured vessels undergo immediate vasoconstriction. Epithelium begins to grow down
along cut edges and along suture
Platelets begin the process of coagulation and initiate Lymphocytes tract. Leukocyte infiltration,
the earliest phase of inflammation. The formation of chiefly round cells (lymphocytes)
the earliest blood clot provides the foundation for with few giant cells, occurs and
future cell migration into the wound. Many inflamma- removes bacteria and necrotic
tory mediators are released during this initial phase. Giant cells tissue.
Once initial homeostasis is achieved, the platelets dis-
charge the contents of their alpha granules into the
extravascular space. Alpha granules contain fibrinogen,
fibronectin, von Willebrand’s factor, factor VIII, and
many other proteins. The fibrinogen is converted into
fibrin, which aids in formation of the fibrin clot. Plate- 5-8 days
lets also play a critical role in releasing growth factors Epithelial downgrowth advances.
and proteases. The best known of these is platelet- Fibroblasts grow in from deeper
derived growth factor (PDGF), which helps mediate tissues and add collagen
the formation of the initial granulation tissue. Fibroblasts precursors and glycoproteins to
matrix. Cellular infiltration
During the late portion of the inflammatory phase, progresses.
leukocytes are seen for the first time. Neutrophils make
up the largest component of the initial leukocyte
response. Neutrophils are drawn into the area by
various cytokines and adhere to the activated vascular
endothelium. They enter the extravascular space by a 10-15 days
process of diapedesis. These early-arriving neutrophils Capillaries grow in from
are responsible for the recruitment of more neutro- Keratinizing subcutaneous tissue, forming
phils, and they also begin the process of killing bacteria pearl granulation tissue. Epithelium
by use of their internal myeloperoxidase system. bridges incision; epithelial
Through the production of free radicals, neutrophils downgrowths regress, leaving
are efficient at killing large numbers of bacteria. Neu- Capillary keratinizing pearls behind.
trophil activity continues for a few days, unless the ingrowth Fibrosed clot (scab) is being
wound is contaminated with bacteria. Once the neutro- pushed out. Collagen formation
phil activity has cleared the wound of bacteria and progresses and cellular
infiltration abates.
other foreign particles, monocytes are recruited into
the wound and activated into macrophages. Macro-
phages are critical in clearing the wound of neutrophils
and any remaining cellular and bacterial debris. 3 weeks–9 months
Macrophages are capable of producing nitrous oxide, Epithelium is thinned to near
which can kill bacteria and has also been shown to normal. Tensile strength of tissue
decrease viral replication. Macrophages also release is increased owing to production
various cytokines, including PDGF, interleukin-6, and and cross-linking of collagen
granulocyte colony-stimulating factor (G-CSF), which fibers; elastic fibers reappear
in turn recruit more monocytes and fibroblasts into the later.
wound.
At this point, new tissue formation, the proliferative
phase of wound healing, has begun. This phase typically
begins on the third day and ends about 14 days after the
initial insult. It is marked by reepithelialization and contain the keratin pairs 5,14 and 6,16. They secrete The final phase of wound healing involves scar matu-
formation of granulation tissue. Reepithelialization vascular endothelial growth factor, which promotes ration and tissue remodeling. This phase overlaps in
occurs by the movement of epithelial cells (keratino- the production of dermal blood vessels. At the same time with the first two phases; it is said to begin with
cytes) from the free edge of the wound slowly across the time the keratinocytes are migrating, the underlying the production of the first granulation tissue. This
wound defect. The migrating cells have the distinct fibroblasts are synthesizing a backbone matrix, made phase extends for months and is complete when most
phenotype of basal keratinocytes. It is believed that up predominantly of type III collagen and some of the collagen III and fibronectin have been replaced
a low calcium concentration in the wound causes proteoglycans. Some of the fibroblasts are converted by mature type I collagen. In the final mature scar, the
the keratinocytes to take on the characteristics of into myofibroblasts by PDGF and tumor growth collagen fibers are oriented in large bundles running
basal keratinocytes. PDGF is an important stimulant for factor-β1. These myofibroblasts are important in that perpendicular to the basement membrane zone. The
keratinocytes and is partially responsible for this migra- they cause the overlying wound to contract, decreasing resulting scar has only 80% of the tensile strength of
tion across the wound. The migrating keratinocytes its surface. the uninjured skin.
THE NETTER COLLECTION OF MEDICAL ILLUSTRATIONS 9