Page 57 - Cardiac Nursing
P. 57
92806_c01.qxd 11/21/11 10:30 AM Page 33
CHAPTER 1 / Cardiac Anatomy and Physiology 33
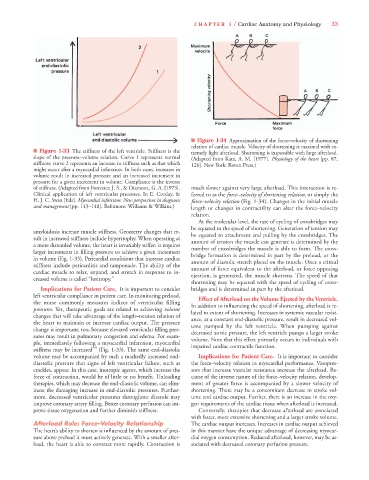
■ Figure 1-34 Approximation of the force–velocity of shortening
relation of cardiac muscle. Velocity of shortening is maximal with ex-
■ Figure 1-33 The stiffness of the left ventricle. Stiffness is the tremely light afterload. Shortening is impossible with large afterload.
slope of the pressure–volume relation. Curve 1 represents normal (Adapted from Katz, A. M. [1977]. Physiology of the heart [pp. 87,
stiffness; curve 2 represents an increase in stiffness such as that which 126]. New York: Raven Press.)
might occur after a myocardial infarction. In both cases, increases in
volume result in increased pressure and an increased increment in
pressure for a given increment in volume. Compliance is the inverse
of stiffness. (Adapted from Forrester, J. S., & Diamone, G. A. [1973]. much slower against very large afterload. This interaction is re-
Clinical application of left ventricular pressures. In E. Corday, & ferred to as the force–velocity of shortening relation, or simply the
H. J. C. Swan [Eds]. Myocardial infarction: New perspectives in diagnosis force–velocity relation (Fig. 1-34). Changes in the initial muscle
and management [pp. 143–148]. Baltimore: Williams & Wilkins.) length or changes in contractility can alter the force–velocity
relation.
At the molecular level, the rate of cycling of crossbridges may
be equated to the speed of shortening. Generation of tension may
amyloidosis increase muscle stiffness. Geometry changes that re- be equated to attachment and pulling by the crossbridges. The
sult in increased stiffness include hypertrophy. When operating at amount of tension the muscle can generate is determined by the
a more distended volume, the heart is invariably stiffer: it requires number of crossbridges the muscle is able to form. The cross-
larger increments in filling pressure to achieve a given increment bridge formation is determined in part by the preload, or the
in volume (Fig. 1-33). Pericardial conditions that increase cardiac amount of diastolic stretch placed on the muscle. Once a critical
stiffness include pericarditis and tamponade. The ability of the amount of force equivalent to the afterload, or force opposing
cardiac muscle to relax, expand, and stretch in response to in- ejection, is generated, the muscle shortens. The speed of that
creased volume is called “lusitropy.”
shortening may be equated with the speed of cycling of cross-
Implications for Patient Care. It is important to consider bridges and is determined in part by the afterload.
left ventricular compliance in patient care. In monitoring preload, Effect of Afterload on the Volume Ejected by the Ventricle.
the nurse commonly measures indices of ventricular filling
pressures. Yet, therapeutic goals are related to achieving volume In addition to influencing the speed of shortening, afterload is re-
lated to extent of shortening. Increases in systemic vascular resist-
changes that will take advantage of the length–tension relation of
ance, at a constant end-diastolic pressure, result in decreased vol-
the heart to maintain or increase cardiac output. The pressure
ume pumped by the left ventricle. When pumping against
change is important, too, because elevated ventricular filling pres-
decreased aortic pressure, the left ventricle pumps a larger stroke
sures may result in pulmonary congestion and edema. For exam-
volume. Note that this effect primarily occurs in individuals with
ple, immediately following a myocardial infarction, myocardial
stiffness may be increased 59 (Fig. 1-33). The same end-diastolic impaired cardiac contractile function.
volume may be accompanied by such a markedly increased end- Implications for Patient Care. It is important to consider
diastolic pressure that signs of left ventricular failure, such as the force–velocity relation in myocardial performance. Vasopres-
crackles, appear. In this case, inotropic agents, which increase the sors that increase vascular resistance increase the afterload. Be-
force of contraction, would be of little or no benefit. Unloading cause of the inverse nature of the force–velocity relation, develop-
therapies, which may decrease the end-diastolic volume, can elim- ment of greater force is accompanied by a slower velocity of
inate the damaging increase in end-diastolic pressures. Further- shortening. There may be a concomitant decrease in stroke vol-
more, decreased ventricular pressures throughout diastole may ume and cardiac output. Further, there is an increase in the oxy-
improve coronary artery filling. Better coronary perfusion can im- gen requirements of the cardiac tissue when afterload is increased.
prove tissue oxygenation and further diminish stiffness. Conversely, therapies that decrease afterload are associated
with faster, more extensive shortening and a larger stroke volume.
Afterload Role: Force–Velocity Relationship The cardiac output increases. Increases in cardiac output achieved
The heart’s ability to shorten is influenced by the amount of pres- in this manner have the unique advantage of decreasing myocar-
sure above preload it must actively generate. With a smaller after- dial oxygen consumption. Reduced afterload, however, may be as-
load, the heart is able to contract more rapidly. Contraction is sociated with decreased coronary perfusion pressure.