Page 584 - Cardiac Nursing
P. 584
6/2
009
6/2
0/0
0/0
1:4
1:4
0
009
0
3
q
q
94.
5-5
94.
3
3
xd
q
xd
3 P
p
p
A
60
A
ara
ara
t
p
t
60
Pa
Pa
M
3 P
M
e 5
e 5
g
g
g
5-5
p
55
55
24_
0-c
K34
LWBK340-c24_
LWB K34 0-c 24_ p pp555-594.qxd 30/06/2009 01:43 PM Page 560 Aptara
LWB
560 PA R T I V / Pathophysiology and Management of Heart Disease
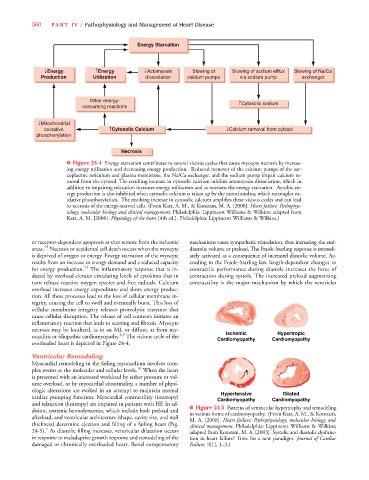
Energy Starvation
↓Energy ↑Energy ↓Actomyosin Slowing of Slowing of sodium efflux Slowing of Na/Ca
Production Utilization dissociation calcium pumps via sodium pump exchanger
Other energy-
↑Cytosolic sodium
consuming reactions
↓Mitochondrial
oxidative ↑Cytosolic Calcium ↓Calcium removal from cytosol
phosphorylation
Necrosis
■ Figure 24-4 Energy starvation contributes to several vicious cycles that cause myocyte necrosis by increas-
ing energy utilization and decreasing energy production. Reduced turnover of the calcium pumps of the sar-
coplasmic reticulum and plasma membrane, the Na/Ca exchanger, and the sodium pump impair calcium re-
moval from the cytosol. The resulting increase in cytosolic calcium inhibits actomyosin dissociation, which in
addition to impairing relaxation increases energy utilization and so worsens the energy starvation. Aerobic en-
ergy production is also inhibited when cytosolic calcium is taken up by the mitochondria, which uncouples ox-
idative phosphorylation. The resulting increase in cytosolic calcium amplifies these vicious cycles and can lead
to necrosis of the energy-starved cells. (From Katz, A. M., & Konstam, M. A. [2008]. Heart failure: Pathophys-
iology, molecular biology and clinical management. Philadelphia: Lippincott Williams & Wilkins; adapted from
Katz, A. M. [2006]. Physiology of the heart [4th ed.]. Philadelphia: Lippincott Williams & Wilkins.)
or receptor-dependent apoptosis at sites remote from the ischemic mechanisms cause sympathetic stimulation, thus increasing the end-
15
areas. Necrosis or accidental cell death occurs when the myocyte diastolic volume or preload. The Frank–Starling response is immedi-
is deprived of oxygen or energy. Energy starvation of the myocyte ately activated as a consequence of increased diastolic volume. Ac-
results from an increase in energy demand and a reduced capacity cording to the Frank–Starling law, length-dependent changes in
for energy production. 11 The inflammatory response that is in- contractile performance during diastole increases the force of
duced by overload elevates circulating levels of cytokines that in contraction during systole. The increased preload augmenting
turn release reactive oxygen species and free radicals. Calcium contractility is the major mechanism by which the ventricles
overload increases energy expenditure and slows energy produc-
tion. All these processes lead to the loss of cellular membrane in-
tegrity, causing the cell to swell and eventually burst. This loss of
cellular membrane integrity releases proteolytic enzymes that
cause cellular disruption. The release of cell contents initiates an
inflammatory reaction that leads to scarring and fibrosis. Myocyte
necrosis may be localized, as in an MI, or diffuse, as from my- Ischemic Hypertropic
ocarditis or idiopathic cardiomyopathy. 6,7 The vicious cycle of the Cardiomyopathy Cardiomyopathy
overloaded heart is depicted in Figure 24-4.
Ventricular Remodeling
Myocardial remodeling in the failing myocardium involves com-
plex events at the molecular and cellular levels. 16 When the heart
is presented with an increased workload by either pressure or vol-
ume overload, or by myocardial abnormality, a number of physi-
ologic alterations are evoked in an attempt to maintain normal Hypertensive Dilated
cardiac pumping function. Myocardial contractility (inotropy) Cardiomyopathy Cardiomyopathy
and relaxation (lusitropy) are impaired in patients with HF. In ad-
dition, systemic hemodynamics, which include both preload and ■ Figure 24-5 Patterns of ventricular hypertrophy and remodeling
in various forms of cardiomyopathy. (From Katz, A. M., & Konstam,
afterload, and ventricular architecture (shape, cavity size, and wall M. A. [2008]. Heart failure: Pathophysiology, molecular biology and
thickness) determine ejection and filling of a failing heart (Fig. clinical management. Philadelphia: Lippincott Williams & Wilkins;
7
24-5). As diastolic filling increases, ventricular dilatation occurs adapted from Konstam, M. A. [2003]. Systolic and diastolic dysfunc-
in response to maladaptive growth response and remodeling of the tion in heart failure? Time for a new paradigm. Journal of Cardiac
damaged or chronically overloaded heart. Renal compensatory Failure, 9[1], 1–3.)