Page 1568 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1568
Chapter 86 Plasma Cell Neoplasms 1395
of MGUS/SMM, which currently does not require therapeutic
intervention, and active symptomatic MM, which needs treatment,
is based on the detection of end-organ damage, which includes bone
lesions, anemia, renal dysfunction, and hypercalcemia. The details of
the diagnostic criteria are listed in Tables 86.7 and 86.8. 20
Radiographic Evaluation
The standard evaluation of bone lesions in myeloma is by a skeletal
survey that includes plain x-rays of the entire skeleton. The presence
of characteristic lytic lesions is considered diagnostic for myeloma
(Fig. 86.8). Rarely, in POEMS, bone lesions are osteosclerotic. The
bone lesions do not always resolve following effective therapy. Because
of the absence or suppression of osteoblastic activity, the bone scan
is diagnostically not a useful investigative tool in myeloma. Almost
all patients with myeloma have osteoporosis as a result of unbalanced
osteoclastic activity. Bone mineral density (BMD) measurement by
dual-energy x-ray absorptiometry helps identify osteoporosis and is
consider a useful investigation. However, it is not uniformly used,
because all patients with myeloma are known to have osteoporosis,
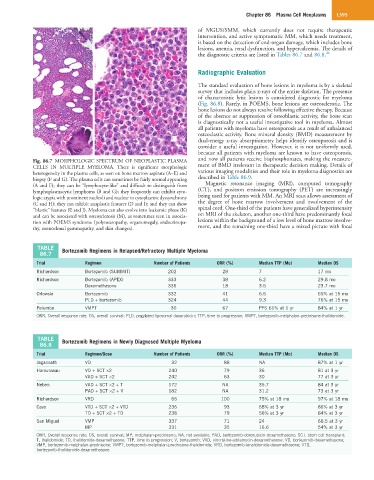
Fig. 86.7 MORPHOLOGIC SPECTRUM OF NEOPLASTIC PLASMA and now all patients receive bisphosphonates, making the measure-
CELLS IN MULTIPLE MYELOMA. There is significant morphologic ment of BMD irrelevant in therapeutic decision making. Details of
heterogeneity in the plasma cells, as seen on bone marrow aspirate (A–E) and various imaging modalities and their role in myeloma diagnostics are
biopsy (F and G). The plasma cells can sometimes be fairly normal appearing described in Table 86.9.
(A and F); they can be “lymphocyte-like” and difficult to distinguish from Magnetic resonance imaging (MRI), computed tomography
lymphoplasmacytic lymphoma (B and G); they frequently can exhibit cyto- (CT), and positron emission tomography (PET) are increasingly
logic atypia with prominent nucleoli and nuclear to cytoplasmic dyssynchrony being used for patients with MM. An MRI scan allows assessment of
(C and H); they can exhibit anaplastic features (D and I); and they can show the degree of bone marrow involvement and involvement of the
“blastic” features (E and J). Myeloma can also evolve into leukemic phase (K) spinal cord. One-third of the patients have generalized hyperintensity
and can be associated with osteosclerosis (M), as sometimes seen in associa- on MRI of the skeleton, another one-third have predominantly focal
tion with POEMS syndrome (polyneuropathy, organomegaly, endocrinopa- lesions within the background of a low level of bone marrow involve-
thy, monoclonal gammopathy, and skin changes). ment, and the remaining one-third have a mixed picture with focal
TABLE Bortezomib Regimens in Relapsed/Refractory Multiple Myeloma
86.7
Trial Regimen Number of Patients ORR (%) Median TTP (Mo) Median OS
Richardson Bortezomib (SUMMIT) 202 28 7 17 mo
Richardson Bortezomib (APEX) 333 38 6.2 29.8 mo
Dexamethasone 336 18 3.5 23.7 mo
Orlowski Bortezomib 332 41 6.5 65% at 15 mo
PLD + bortezomib 324 44 9.3 76% at 15 mo
Palumbo VMPT 30 67 PFS 61% at 1 yr 84% at 1 yr
ORR, Overall response rate; OS, overall survival; PLD, pegylated liposomal doxorubicin; TTP, time to progression; VMPT, bortezomib-melphalan-prednisone-thalidomide.
TABLE Bortezomib Regimens in Newly Diagnosed Multiple Myeloma
86.8
Trial Regimen/Dose Number of Patients ORR (%) Median TTP (Mo) Median OS
Jagannath VD 32 88 NA 87% at 1 yr
Harousseau VD + SCT ×2 240 79 36 81 at 3 yr
VAD + SCT ×2 242 63 30 77 at 3 yr
Neben VAD + SCT ×2 + T 172 NA 35.7 84 at 3 yr
PAD + SCT ×2 + V 182 NA 31.2 73 at 3 yr
Richardson VRD 66 100 75% at 18 mo 97% at 18 mo
Cavo VTD + SCT ×2 + VTD 236 93 68% at 3 yr 86% at 3 yr
TD + SCT ×2 + TD 238 79 56% at 3 yr 84% at 3 yr
San Miguel VMP 337 71 24 68.5 at 3 yr
MP 331 35 16.6 54% at 3 yr
ORR, Overall response rate; OS, overall survival; MP, melphalan-prednisone; NA, not available; PAD, bortezomib-doxorubicin-dexamethasone; SCT, stem cell transplant;
T, thalidomide; TD, thalidomide-dexamethasone; TTP, time to progression; V, bortezomib; VAD, vincristine-adriamycin-dexamethasone; VD, bortezomib-dexamethasone;
VMP, bortezomib-melphalan-prednisone; VMPT, bortezomib-melphalan-prednisone-thalidomide; VRD, bortezomib-lenalidomide-dexamethasone; VTD,
bortezomib-thalidomide-dexamethasone.