Page 1573 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1573
1400 Part VII Hematologic Malignancies
100 100 MRD-negative
MRD-negative N= 599
N = 660 MRD-positive
MRD-positive N= 501
80 χ =134.4 80 χ =25.27
2
2
N = 613 1 1
P >.00001 P = .0000005
% PFS 60 cumulative % surviving 60
40 40
20 20
0 2 4 6 8 10 12 0 2 4 6 8 10 12
Time (years) Time (years)
No. at risk: No. at risk:
MRD negative: 457 214 70 12 1 508 359 139 26 4
MRD positive: 308 113 28 4 1 390 250 105 17 5
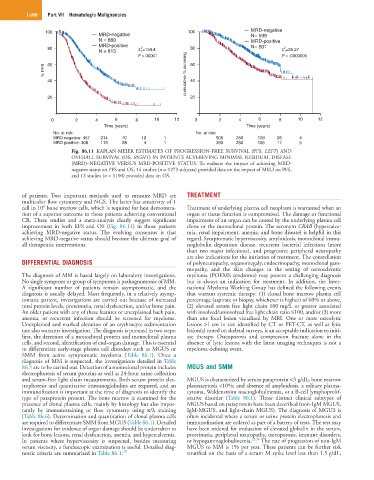
Fig. 86.11 KAPLAN-MEIER ESTIMATES OF PROGRESSION-FREE SURVIVAL (PFS; LEFT) AND
OVERALL SURVIVAL (OS; RIGHT) IN PATIENTS ACVHIEVING MINIMAL RESIDUAL DISEASE
(MRD)–NEGATIVE VERSUS MRD-POSITIVE STATUS. To evaluate the impact of achieving MRD-
negative status on PFS and OS, 14 studies (n = 1273 subjects) provided data on the impact of MRD on PFS,
and 12 studies (n = 1100) provided data on OS.
of patients. Two important methods used to measure MRD are TREATMENT
multicolor flow cytometry and NGS. The latter has sensitivity of 1
6
cell in 10 bone marrow cells, which is required for best demonstra- Treatment of underlying plasma cell neoplasm is warranted when an
tion of a superior outcome in those patients achieving conventional organ or tissue function is compromised. The damage or functional
CR. These studies and a meta-analysis clearly suggest significant impairment of an organ can be caused by the underlying plasma cell
improvement in both EFS and OS (Fig. 86.11) in those patients clone or the monoclonal protein. The acronym CRAB (hypercalce-
achieving MRD-negative status. The evolving consensus is that mia, renal impairment, anemia, and bone disease) is helpful in this
achieving MRD-negative status should become the ultimate goal of regard. Symptomatic hyperviscosity, amyloidosis, monoclonal immu-
all therapeutic interventions. noglobulin deposition disease, recurrent bacterial infections (more
than two major infections), and progressive peripheral neuropathy
are also indications for the initiation of treatment. The constellation
DIFFERENTIAL DIAGNOSIS of polyneuropathy, organomegaly, endocrinopathy, monoclonal gam-
mopathy, and the skin changes in the setting of osteosclerotic
The diagnosis of MM is based largely on laboratory investigations. myeloma (POEMS syndrome) may present a challenging diagnosis
No single symptom or group of symptoms is pathognomonic of MM. but is always an indication for treatment. In addition, the Inter-
A significant number of patients remain asymptomatic, and the national Myeloma Working Group has defined the following events
diagnosis is usually delayed. Most frequently, in a relatively asymp- that warrant systemic therapy: (1) clonal bone marrow plasma cell
tomatic patient, investigations are carried out because of increased percentage (aspirate or biopsy, whichever is higher) of 60% or above,
total protein levels, proteinuria, renal dysfunction, and/or bone pain. (2) elevated serum free light chain 100 mg/L or greater associated
An older patient with any of these features or unexplained back pain, with involved/uninvolved free light chain ratio ≤100, and/or (3) more
anemia, or recurrent infection should be screened for myeloma. than one focal lesion visualized by MRI. One or more osteolytic
Unexplained and marked elevation of an erythrocyte sedimentation lesions >1 cm in size identified by CT or PET-CT, as well as lytic
rate also warrants investigation. The diagnosis is pursued in two steps: lesion(s) noted on skeletal surveys, is an acceptable indication to initi-
first, the detection of a monoclonal protein and monoclonal plasma ate therapy. Osteoporosis and compression fracture alone in the
cells, and second, identification of end-organ damage. This is essential absence of lytic lesions with the latest imaging techniques is not a
to differentiate early-stage plasma cell disorders such as MGUS or myeloma-defining event.
SMM from active symptomatic myeloma (Table 86.1). Once a
diagnosis of MM is suspected, the investigations detailed in Table
86.5 are to be carried out. Detection of a monoclonal protein includes MGUS and SMM
electrophoresis of serum proteins as well as 24-hour urine collection
and serum-free light chain measurements. Both serum protein elec- MGUS is characterized by serum paraprotein <3 g/dL; bone marrow
trophoresis and quantitative immunoglobulins are required, and an plasmacytosis <10%; and absence of amyloidosis, a solitary plasma-
immunofixation is important at the time of diagnosis to identify the cytoma, Waldenström macroglobulinemia, or a B-cell lymphoprolif-
type of paraprotein present. The bone marrow is examined for the erative disorder (Table 86.1). Three distinct clinical subtypes of
presence of clonal plasma cells, mainly by histology but also impor- MGUS based on paraprotein have been described (non-IgM MGUS,
tantly by immunostaining or flow cytometry using κ/λ staining IgM-MGUS, and light-chain MGUS). The diagnosis of MGUS is
(Table 86.6). Determination and quantitation of clonal plasma cells often incidental where a serum or urine protein electrophoresis and
are required to differentiate SMM from MGUS (Table 86.1). Detailed immunofixation are ordered as part of a battery of tests. The test may
investigations for evidence of organ damage should be undertaken to have been ordered for evaluation of elevated globulin in the serum,
look for bony lesions, renal dysfunction, anemia, and hypercalcemia. proteinuria, peripheral neuropathy, osteoporosis, immune disorders,
In patients where hyperviscosity is suspected, besides measuring or hypogammaglobulinemia. 25,26 The rate of progression of non-IgM
serum viscosity, a funduscopic examination is useful. Detailed diag- MGUS to MM is 1% per year. These patients can be further risk
nostic criteria are summarized in Table 86.1. 20 stratified on the basis of a serum M spike level less than 1.5 g/dL,