Page 1577 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1577
1404 Part VII Hematologic Malignancies
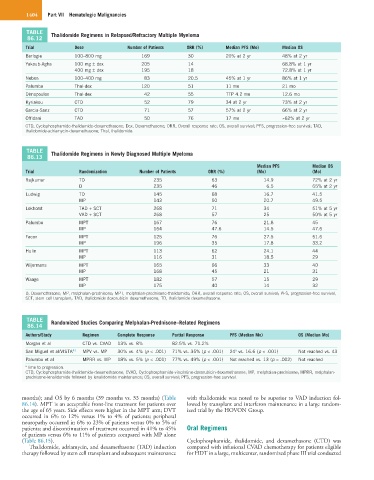
TABLE Thalidomide Regimens in Relapsed/Refractory Multiple Myeloma
86.12
Trial Dose Number of Patients ORR (%) Median PFS (Mo) Median OS
Barlogie 100–800 mg 169 30 20% at 2 yr 48% at 2 yr
Yakoub-Agha 100 mg ± dex 205 14 68.8% at 1 yr
400 mg ± dex 195 18 72.8% at 1 yr
Neben 100–400 mg 83 20.5 45% at 1 yr 86% at 1 yr
Palumbo Thal-dex 120 51 11 mo 21 mo
Dimopoulos Thal-dex 42 55 TTP 4.2 mo 12.6 mo
Kyriakou CTD 52 79 34 at 2 yr 73% at 2 yr
Garcia-Sanz CTD 71 57 57% at 2 yr 66% at 2 yr
Offidani TAD 50 76 17 mo ≈62% at 2 yr
CTD, Cyclophosphamide-thalidomide-dexamethasone; Dex, Dexamethasone; ORR, Overall response rate; OS, overall survival; PFS, progression-free survival; TAD,
thalidomide-adriamycin-dexamethasone; Thal, thalidomide.
TABLE Thalidomide Regimens in Newly Diagnosed Multiple Myeloma
86.13
Median PFS Median OS
Trial Randomization Number of Patients ORR (%) (Mo) (Mo)
Rajkumar TD 235 63 14.9 72% at 2 yr
D 235 46 6.5 65% at 2 yr
Ludwig TD 145 68 16.7 41.5
MP 143 50 20.7 49.5
Lokhorst TAD + SCT 268 71 34 51% at 5 yr
VAD + SCT 268 57 25 50% at 5 yr
Palumbo MPT 167 76 21.8 45
MP 164 47.6 14.5 47.6
Facon MPT 125 76 27.5 51.6
MP 196 35 17.8 33.2
Hulin MPT 113 62 24.1 44
MP 116 31 18.5 29
Wijermans MPT 165 66 33 40
MP 168 45 21 31
Waage MPT 182 57 15 29
MP 175 40 14 32
D, Dexamethasone; MP, melphalan-prednisone; MPT, melphalan-prednisone-thalidomide; ORR, overall response rate; OS, overall survival; PFS, progression-free survival,
SCT, stem cell transplant; TAD, thalidomide-doxorubicin-dexamethasone; TD, thalidomide-dexamethasone.
TABLE Randomized Studies Comparing Melphalan-Prednisone–Related Regimens
86.14
Authors/Study Regimen Complete Response Partial Response PFS (Median Mo) OS (Median Mo)
Morgan et al CTD vs. CVAD 13% vs. 8% 82.5% vs. 71.2%
a
San Miguel et al/VISTA 41 MPV vs. MP 30% vs. 4% (p < .001) 71% vs. 35% (p < .001) 24 vs. 16.6 (p < .001) Not reached vs. 43
Palumbo et al MPRR vs. MP 18% vs. 5% (p < .001) 77% vs. 49% (p < .001) Not reached vs. 13 (p = .002) Not reached
a Time to progression.
CTD, Cyclophosphamide-thalidomide-dexamethasone; CVAD, Cyclophosphamide-vincristine-doxorubicin-dexamethasone; MP, melphalan-prednisone; MPRR, melphalan-
prednisone-lenalidomide followed by lenalidomide maintenance; OS, overall survival; PFS, progression-free survival.
months); and OS by 6 months (39 months vs. 33 months) (Table with thalidomide was noted to be superior to VAD induction fol-
86.14). MPT is an acceptable front-line treatment for patients over lowed by transplant and interferon maintenance in a large random-
the age of 65 years. Side effects were higher in the MPT arm; DVT ized trial by the HOVON Group.
occurred in 6% to 12% versus 1% to 4% of patients; peripheral
neuropathy occurred in 6% to 23% of patients versus 0% to 5% of
patients; and discontinuation of treatment occurred in 41% to 45% Oral Regimens
of patients versus 6% to 11% of patients compared with MP alone
(Table 86.15). Cyclophosphamide, thalidomide, and dexamethasone (CTD) was
Thalidomide, adriamycin, and dexamethasone (TAD) induction compared with infusional CVAD chemotherapy for patients eligible
therapy followed by stem cell transplant and subsequent maintenance for HDT in a large, multicenter, randomized phase III trial conducted