Page 1574 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1574
Chapter 86 Plasma Cell Neoplasms 1401
100 100 No t(4;14)/ 100
del(17p) No t(4;14)/
75 No t(4;14)/ 75 75 del(17p)
Patients (%) 50 t(4;14)/ Patients (%) 50 del(17p)/ Patients (%) 50
del(17p)
25
P <.001 del(17p) 25 P <.001 t(4;14) 25 P = .001 del(17p)/
t(4;14)
20 40 60 20 40 60 20 40 60
ISS 1 (mo) ISS 2 (mo) ISS 3 (mo)
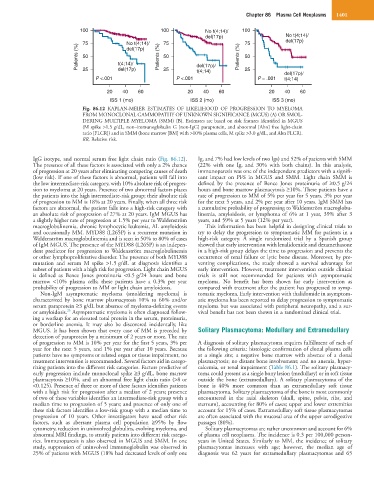
Fig. 86.12 KAPLAN-MEIER ESTIMATES OF LIKELIHOOD OF PROGRESSION TO MYELOMA
FROM MONOCLONAL GAMMOPATHY OF UNKNOWN SIGNIFICANCE (MGUS) (A) OR SMOL-
DERING MULTIPLE MYELOMA (SMM) (B). Estimates are based on risk features identified in MGUS
(M spike >1.5 g/dL, non–immunoglobulin G [non-IgG] paraprotein, and abnormal [Abn] free light-chain
ratio [FLCR]) and in SMM (bone marrow [BM] with >10% plasma cells, M spike >3.0 g/dL, and Abn FLCR).
RR, Relative risk.
IgG isotype, and normal serum free light chain ratio (Fig. 86.12). Ig, and 7% had low levels of two Igs) and 52% of patients with SMM
The presence of all these factors is associated with only a 2% chance (22% with one Ig, and 30% with both chains). In this analysis,
of progression at 20 years after eliminating competing causes of death immunoparesis was one of the independent predictors with a signifi-
(low risk). If one of these factors is abnormal, patients will fall into cant impact on PFS in MGUS and SMM. Light chain SMM is
the low intermediate-risk category, with 10% absolute risk of progres- defined by the presence of Bence Jones proteinuria of ≥0.5 g/24
sion to myeloma at 20 years. Presence of two abnormal factors places hours and bone marrow plasmacytosis ≥10%. These patients have a
the patients into the high intermediate-risk group; their absolute risk rate of progression to MM of 5% per year for 5 years, 3% per year
of progression to MM is 18% at 20 years. Finally, when all three risk for the next 5 years, and 2% per year after 10 years. IgM SMM has
factors are abnormal, the patient falls into a high-risk category with a cumulative probability of progressing to Waldenström macroglobu-
an absolute risk of progression of 27% at 20 years. IgM MGUS has linemia, amyloidosis, or lymphoma of 6% at 1 year, 39% after 3
a slightly higher rate of progression at 1.5% per year to Waldenström years, and 59% at 5 years (12% per year).
macroglobulinemia, chronic lymphocytic leukemia, AL amyloidosis This information has been helpful in designing clinical trials to
and occasionally MM. MYD88 (L265P) is a recurrent mutation in try to delay the progression to symptomatic MM for patients in a
Waldenström macroglobulinemia and is seen in 50% to 80% of cases high-risk category. A single randomized trial by a Spanish group
of IgM MGUS. The presence of the MYD88 (L265P) is an indepen- showed that early intervention with lenalidomide and dexamethasone
dent predictor for progression to Waldenström macroglobulinemia in a high-risk group delays the time to progression and prevents the
or other lymphoproliferative disorder. The presence of both MYD88 occurrence of renal failure or lytic bone disease. Moreover, by pre-
mutation and serum M spike >1.5 g/dL at diagnosis identifies a venting complications, the study showed a survival advantage for
subset of patients with a high risk for progression. Light chain MGUS early intervention. However, treatment intervention outside clinical
is defined as Bence Jones proteinuria <0.5 g/24 hours and bone trials is still not recommended for patients with asymptomatic
marrow <10% plasma cells; these patients have a 0.3% per year myeloma. No benefit has been shown for early intervention as
probability of progression to MM or light chain amyloidosis. compared with treatment after the patient has progressed to symp-
Non-IgM asymptomatic myeloma (smoldering myeloma) is tomatic myeloma. Early intervention with thalidomide in asymptom-
characterized by bone marrow plasmacytosis 10% to 60% and/or atic myeloma has been reported to delay progression to symptomatic
serum paraprotein ≥3 g/dL but absence of myeloma-defining events myeloma but was associated with peripheral neuropathy, and a sur-
25
or amyloidosis. Asymptomatic myeloma is often diagnosed follow- vival benefit has not been shown in a randomized clinical trial.
ing a workup for an elevated total protein in the serum, proteinuria,
or borderline anemia. It may also be discovered incidentally, like
MGUS. It has been shown that every case of MM is preceded by Solitary Plasmacytoma: Medullary and Extramedullary
detection of paraprotein by a minimum of 2 years or more. The rate
of progression to MM is 10% per year for the first 5 years, 3% per A diagnosis of solitary plasmacytoma requires fulfillment of each of
year for the next 5 years, and 1% per year after 10 years. Because the following criteria: histologic confirmation of clonal plasma cells
patients have no symptoms or related organ or tissue impairment, no at a single site; a negative bone marrow with absence of a clonal
treatment intervention is recommended. Several factors aid in catego- plasmacytosis; no distant bone involvement; and no anemia, hyper-
rizing patients into the different risk categories. Factors predictive of calcemia, or renal impairment (Table 86.1). The solitary plasmacy-
early progression include monoclonal spike ≥3 g/dL, bone marrow toma could present as a single bony lesion (medullary) or in soft tissue
plasmacytosis ≥10%, and an abnormal free light chain ratio (>8 or outside the bone (extramedullary). A solitary plasmacytoma of the
<0.125). Presence of three or more of these factors identifies patients bone is 40% more common than an extramedullary soft tissue
with a high risk for progression after a median of 2 years; presence plasmacytoma. Solitary plasmacytoma of the bone is most commonly
of two of these variables identifies an intermediate-risk group with a encountered in the axial skeleton (skull, spine, pelvis, ribs, and
median time to progression of 5 years; and presence of only one of sternum), accounting for 80% of cases; upper and lower extremities
these risk factors identifies a low-risk group with a median time to account for 15% of cases. Extramedullary soft tissue plasmacytomas
progression of 10 years. Other investigators have used other risk are often associated with the mucosal area of the upper aerodigestive
factors, such as aberrant plasma cell population ≥95% by flow passages (80%).
cytometry, reduction in uninvolved globulins, evolving myeloma, and Solitary plasmacytomas are rather uncommon and account for 6%
abnormal MRI findings, to stratify patients into different risk catego- of plasma cell neoplasms. The incidence is 0.3 per 100,000 person-
ries. Immunoparesis is also observed in MGUS and SMM. In one years in United States. Similarly to MM, the incidence of solitary
study, suppression of uninvolved immunoglobulin was observed in plasmacytomas increases with age; however, the median age of
25% of patients with MGUS (18% had decreased levels of only one diagnosis was 62 years for extramedullary plasmacytomas and 65